SUMMARY
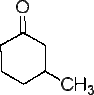
The discussion centers on identifying the stereogenic center in 3-methylcyclohexanone. Participants clarify that the carbon atom bonded to the methyl group is indeed a stereogenic center, despite initial reasoning suggesting otherwise. The key factor in determining chirality is the uniqueness of the substituents attached to the carbon, which includes hydrogen and two methylene groups. The conversation also raises the question of whether additional stereogenic centers exist at ortho and para positions in the compound.
PREREQUISITES
- Understanding of stereochemistry and chirality
- Familiarity with cyclohexanone structures
- Knowledge of substituent effects on carbon atoms
- Basic principles of organic chemistry
NEXT STEPS
- Study the concept of chirality in organic compounds
- Explore stereogenic centers in cyclic structures
- Research the effects of substituents on carbon atom chirality
- Investigate the presence of multiple stereogenic centers in substituted cycloalkanes
USEFUL FOR
Chemistry students, organic chemists, and educators seeking to deepen their understanding of stereochemistry and the identification of chiral centers in organic molecules.