aaaa202
- 1,144
- 2
I want to calculate the surface energy for the (001) plane in a simple cubic lattice. My idea is this:
When I cleave a simple cubic crystal I create 2 surfaces each sharing an amount of broken bonds. I want to find the amount of broken bonds per area, because I can associate an energy with these broken bonds. I associate both an energy for the broken bonds for first nearest neighbours, second nearest neighbours and third nearest neighbours. If the lattice constant is a there is one atom per area a^2. So I find the energy corresponding to the broken bond of this atom and divide by 2*a^2 (because I create 2 surfaces).
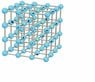
Where I think I go wrong is finding the amount of nearest neighbours. For first nearest neighbours there is only one. For the second I count 5 since for the atoms between which a bond has been broken there are 5 other atoms being first neighbours. And the third nearest neighbour broken bonds can be counted with the same idea. However this is not at all what my book gets (author gets 1,4,4. Where am I misunderstanding something? I have attached a sketch of my counting logic. The bonds are counted with respect to the red dotted atoms, and the green is first nearest neighbour, yellow second and black third (though the drawing doesn't include all of the these)
Is this even the correct way to go about the problem?
When I cleave a simple cubic crystal I create 2 surfaces each sharing an amount of broken bonds. I want to find the amount of broken bonds per area, because I can associate an energy with these broken bonds. I associate both an energy for the broken bonds for first nearest neighbours, second nearest neighbours and third nearest neighbours. If the lattice constant is a there is one atom per area a^2. So I find the energy corresponding to the broken bond of this atom and divide by 2*a^2 (because I create 2 surfaces).
Where I think I go wrong is finding the amount of nearest neighbours. For first nearest neighbours there is only one. For the second I count 5 since for the atoms between which a bond has been broken there are 5 other atoms being first neighbours. And the third nearest neighbour broken bonds can be counted with the same idea. However this is not at all what my book gets (author gets 1,4,4. Where am I misunderstanding something? I have attached a sketch of my counting logic. The bonds are counted with respect to the red dotted atoms, and the green is first nearest neighbour, yellow second and black third (though the drawing doesn't include all of the these)
Is this even the correct way to go about the problem?