SUMMARY
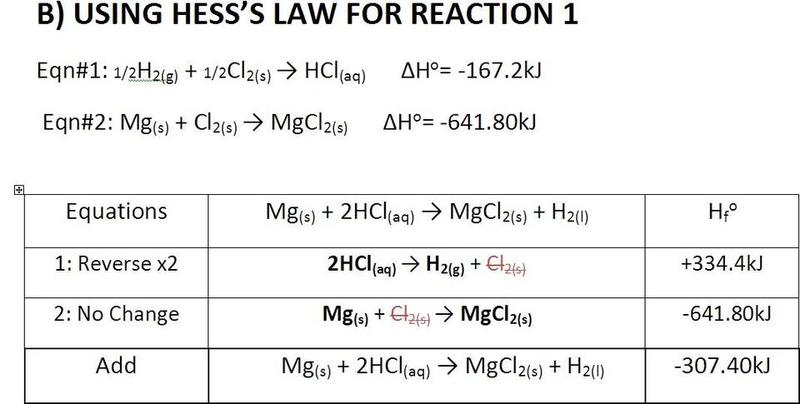
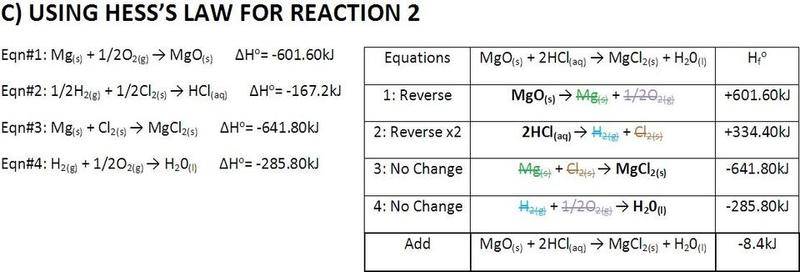
The forum discussion centers on the calculation of enthalpy for magnesium oxide (MgO) and hydrochloric acid (HCl) using Hess's Law. A user seeks validation of their calculations, expressing concern over the accuracy of their results. Another participant confirms the calculations are mostly correct but suggests that the second answer should include one additional significant figure. They also recommend consulting standard enthalpy tables for accurate values, providing a specific online resource for further reference.
PREREQUISITES
- Understanding of Hess's Law
- Familiarity with enthalpy calculations
- Knowledge of significant figures in scientific measurements
- Access to standard enthalpy tables or databases
NEXT STEPS
- Research the application of Hess's Law in thermodynamics
- Learn how to accurately determine significant figures in calculations
- Explore online resources for standard enthalpy values, such as the provided link
- Study the enthalpy changes for common reactions involving MgO and HCl
USEFUL FOR
Chemistry students, educators, and professionals involved in thermodynamics and enthalpy calculations will benefit from this discussion.