SUMMARY
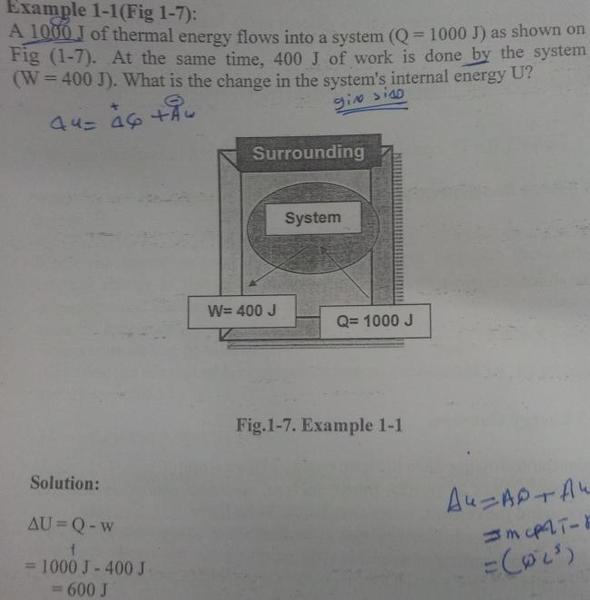
The discussion focuses on the sign convention for work in thermodynamics, specifically how to determine whether work is positive or negative. When a system does work on its surroundings, it is considered negative, while work done on the system is positive. This is illustrated through the first law of thermodynamics, represented as ΔU = Q - W. For example, if 400 J of work is done by the system, it results in a decrease in internal energy, while negative work indicates energy gain for the system.
PREREQUISITES
- Understanding of the first law of thermodynamics (ΔU = Q - W)
- Familiarity with concepts of work and energy in physics
- Knowledge of sign conventions in thermodynamic processes
- Basic grasp of heat transfer and internal energy
NEXT STEPS
- Study the implications of the first law of thermodynamics in various scenarios
- Explore examples of work done by and on a system in thermodynamic processes
- Learn about different types of thermodynamic systems and their energy exchanges
- Investigate the relationship between heat transfer and work in closed systems
USEFUL FOR
Students of physics, engineers working with thermodynamic systems, and anyone seeking to understand energy transfer and work in thermodynamics.