Discussion Overview
The discussion revolves around the concept of alveolar surface tension, particularly the forces at play at the air-liquid interface within alveoli. Participants explore the implications of surface tension on alveolar size and the behavior of water molecules in this context, touching on both physiological and physical principles.
Discussion Character
- Exploratory
- Technical explanation
- Conceptual clarification
- Debate/contested
Main Points Raised
- One participant expresses confusion about how the force that pushes water molecules toward cell walls can also create a force that pushes in the opposite direction.
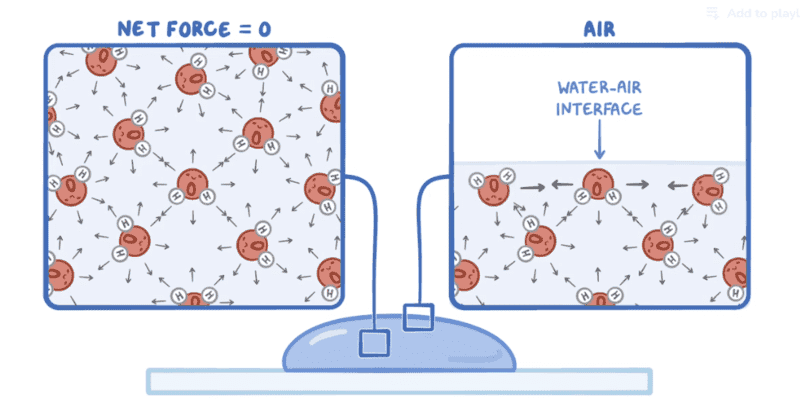
- Another participant suggests comparing two scenarios to understand the net effects of forces acting on the alveolar surface, rather than analyzing them in isolation.
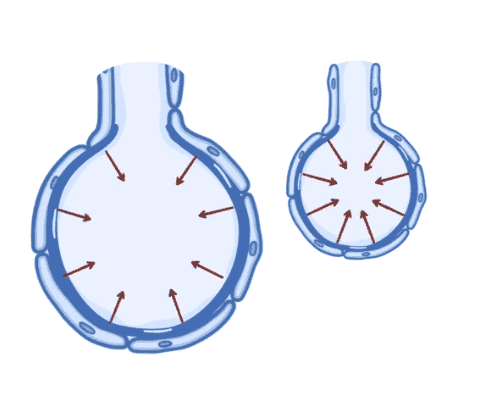
- A medical student questions the textbook explanation of surface tension, arguing that if surface tension pulls water molecules together, it should lead to alveolar expansion rather than contraction.
- Some participants explain that surface tension behaves similarly to a balloon, where the surface "wants" to minimize its area, leading to a compressive force on the air inside the alveoli.
- One participant introduces the concept of water molecules as dipoles and discusses how external atmospheric pressure distorts these dipoles, creating forces that contribute to surface tension and affect the alveolar structure.
- The same participant elaborates on how the arrangement of dipoles leads to both horizontal and vertical forces, with the vertical components contributing to an upward force that opposes external pressure.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the explanation of alveolar surface tension, with multiple competing views and interpretations of the forces involved remaining present throughout the discussion.
Contextual Notes
Some claims rely on specific assumptions about the behavior of water molecules and the nature of forces at play, which are not fully resolved in the discussion. The relationship between surface tension and alveolar dynamics remains complex and is subject to differing interpretations.