Sorade
- 53
- 1
Hi all,
I need to estimate the mechanical work I can recover from expanding hot air through a gas turbine.
So far I am using the equation below, where Wrev is my ideal isentropic work, s the number of stages, n = k = ratio of specific heats, R gas constant, T1 is the inlet Temperature, Pin the inlet pressure, Pout the outlet pressure.
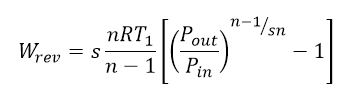
This equation was derived assuming an ideal gas was used. I would like to know how valid this is when the inlet conditions are T = 600 K and P = 250 bars, and outlet conditions T = 350 K and P = 1 bar.
I am asking because I saw this simplification used in many papers and I want to know what impact that might have on my estimations. I assume an isentropic efficiency between 0.66 and 0.88 and calculated all the work outcomes for the values in this range.
I need to estimate the mechanical work I can recover from expanding hot air through a gas turbine.
So far I am using the equation below, where Wrev is my ideal isentropic work, s the number of stages, n = k = ratio of specific heats, R gas constant, T1 is the inlet Temperature, Pin the inlet pressure, Pout the outlet pressure.
This equation was derived assuming an ideal gas was used. I would like to know how valid this is when the inlet conditions are T = 600 K and P = 250 bars, and outlet conditions T = 350 K and P = 1 bar.
I am asking because I saw this simplification used in many papers and I want to know what impact that might have on my estimations. I assume an isentropic efficiency between 0.66 and 0.88 and calculated all the work outcomes for the values in this range.