Discussion Overview
The discussion centers on the differences between two graphs depicting Gibbs free energy and activation energy in chemical reactions. Participants explore the implications of these graphs for understanding reaction spontaneity, kinetics, and thermodynamics.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Conceptual clarification
Main Points Raised
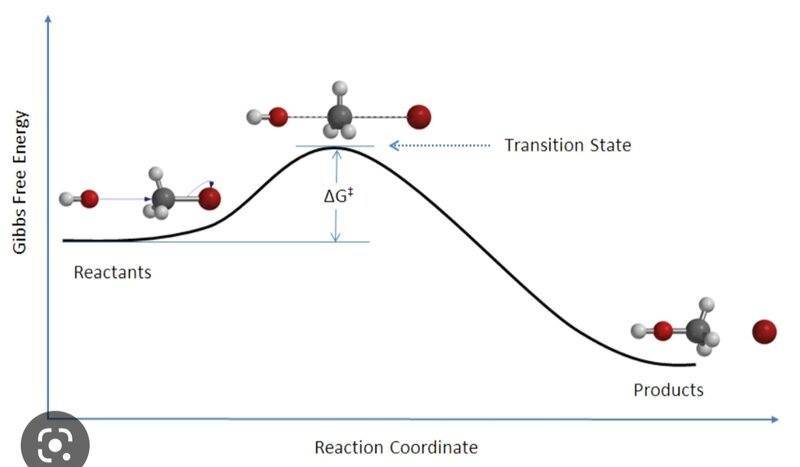
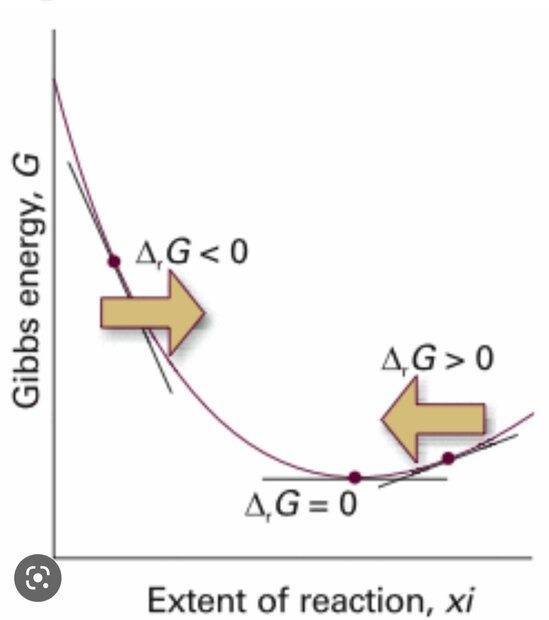
- One participant questions why Gibbs free energy reaches a minimum in one graph and a maximum in another, suggesting that a chemical reaction should always have an energy maximum represented by activation energy.
- Another participant notes that the graphs depict different scenarios, emphasizing that one graph deals with changes rather than point values.
- It is proposed that the first graph highlights the Gibbs energy necessary for the transformation of reactants into products, while the second graph reflects the Gibbs energy of the reaction mixture at various extents of reaction.
- Some participants clarify that the "system" in the first case refers to a pair of reacting molecules, while in the second case, it refers to an ensemble of molecules, indicating a distinction between reaction coordinate and extent of reaction.
- There is a discussion about the relationship between Gibbs free energy and activation energy, with one participant suggesting that they may not be correlated, while another asks for clarification on their relationship.
- One participant asserts that the second plot is purely thermodynamic and does not reference kinetics, while another agrees that the Gibbs free energy of activation is related to the speed of the reaction.
- A later reply questions whether the reaction can be defined as exergonic or endergonic solely by examining the thermodynamic plot, raising concerns about the relationship between the two graphs.
Areas of Agreement / Disagreement
Participants express differing views on the relationship between Gibbs free energy and activation energy, with no consensus reached on whether they are correlated. The discussion remains unresolved regarding the implications of the thermodynamic plot for defining reaction spontaneity.
Contextual Notes
Participants highlight that the terms "reaction coordinate" and "extent of reaction" are distinct and that the second plot does not address kinetics. There are also unresolved questions about the definitions and implications of exergonic and endergonic reactions in relation to the graphs.