r12214001
- 24
- 2
- Homework Statement
- energy conversion question
- Relevant Equations
- calculate temperature change
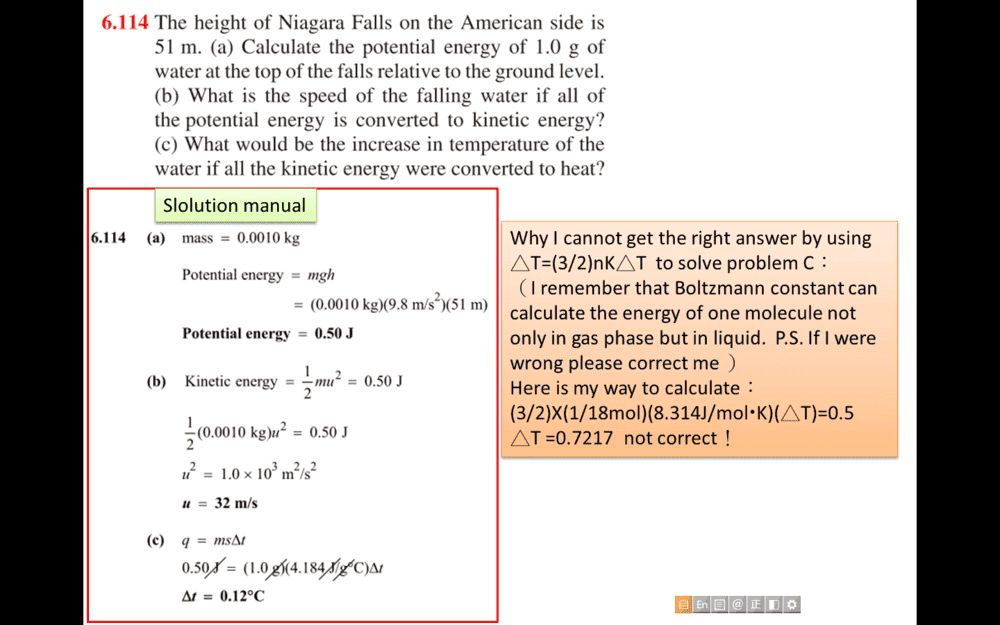
Why I cannot get the right answer by using △T=(3/2)nK△T to solve problem C:
Work done at constant pressure is always ##P\Delta V##. And since enthalpy is defined as: H = U + PV, it follows that ##\Delta H = \Delta U + P\Delta V + V\Delta P = \Delta U + P\Delta V## at constant pressure.r12214001 said:As you explaned, W=NKT can only be used in ideal gas.
Why the work for solid Graphite and diamond can be calculated by PV? Because NKT=nRT=PV
concept corrected TKSAndrew Mason said:Work done at constant pressure is always ##P\Delta V##. And since enthalpy is defined as: H = U + PV, it follows that ##\Delta H = \Delta U + P\Delta V + V\Delta P = \Delta U + P\Delta V## at constant pressure.
AM