SUMMARY
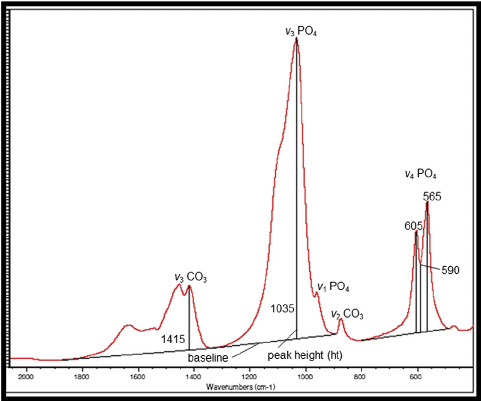
The discussion focuses on the interpretation of FTIR (Fourier Transform Infrared Spectroscopy) peaks for PO4 (phosphate) compounds. It establishes that multiple peaks at different wavelengths arise from various vibrational modes of the molecule, including stretching and bending vibrations. Specifically, the modes v1, v3, and v4 correspond to distinct types of molecular vibrations, such as symmetric stretching and asymmetric stretching. Understanding these peaks is crucial for accurately identifying and analyzing phosphate compounds in various applications.
PREREQUISITES
- Basic knowledge of FTIR spectroscopy principles
- Understanding of molecular vibrations and their classifications
- Familiarity with the structure of phosphate compounds
- Experience with interpreting IR spectra
NEXT STEPS
- Research the specific vibrational modes of PO4 compounds in FTIR analysis
- Learn about the classification of molecular vibrations: stretching, bending, rocking, and wagging
- Explore advanced FTIR techniques for analyzing complex mixtures
- Study the impact of molecular structure on FTIR peak patterns
USEFUL FOR
Chemists, materials scientists, and researchers involved in spectroscopy or the analysis of phosphate compounds will benefit from this discussion.