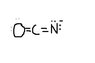SUMMARY
The discussion centers on determining the most significant Lewis structure for the OCH- ion, emphasizing the assignment of formal charges. Participants clarify that oxygen (O) is more electronegative than nitrogen (N), which justifies the negative charge residing on the oxygen atom. The conversation also highlights the importance of varying bond orders to explore different structural possibilities. A correction is noted regarding the notation of the ion, confirming it as OCH-.
PREREQUISITES
- Understanding of Lewis structures and formal charge calculations
- Knowledge of electronegativity and its implications in molecular structures
- Familiarity with bond order concepts in chemistry
- Basic skills in chemical notation and ion representation
NEXT STEPS
- Research the principles of formal charge assignment in molecular structures
- Explore the concept of electronegativity and its role in determining charge distribution
- Learn about bond order variations and their effects on molecular stability
- Study the Lewis structures of other common ions for comparative analysis
USEFUL FOR
Chemistry students, educators, and anyone involved in molecular modeling or structural chemistry analysis.