Bolter
- 262
- 31
- Homework Statement
- Calculate the metal's work function
- Relevant Equations
- See below
Question:

I have tried this and got work function to e 5.1eV
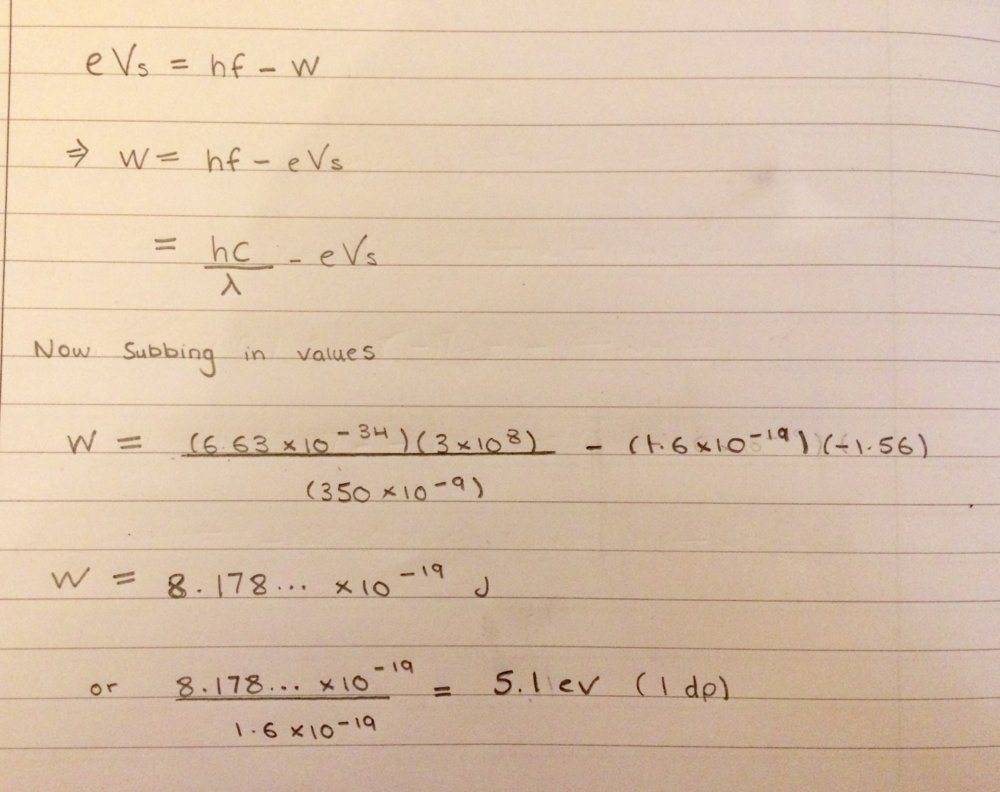
My concern is that for these type of questions, do I need to take into account the signs of some values; such as the negative sign for the charge of an electron? Or could I just take the magnitude for all the values
Any help would be appreciated! Thanks
I have tried this and got work function to e 5.1eV
My concern is that for these type of questions, do I need to take into account the signs of some values; such as the negative sign for the charge of an electron? Or could I just take the magnitude for all the values
Any help would be appreciated! Thanks
