Discussion Overview
The discussion revolves around the pH of alanine salt, specifically addressing its dissociation in solution, the behavior of its functional groups, and the implications for its acidity or basicity. Participants explore concepts related to amino acids, zwitterions, and potential polymerization in the context of alanine.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Conceptual clarification
Main Points Raised
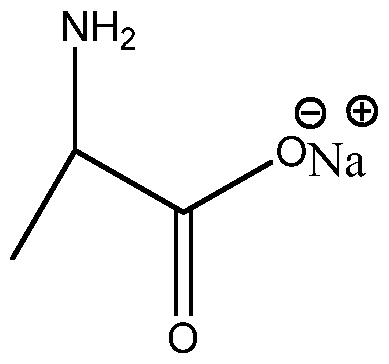
- Some participants question whether alanine salt dissociates in solution and how this affects its pH, noting that it has a pH of about 6.113, which is suggested to be the average of its two pKa values.
- There is a discussion about the carboxyl group potentially acting as a weak base and whether the amine group can be considered a weak acid, with some uncertainty expressed about the terminology used.
- One participant asserts that sodium alanine salt will not have a pH near 6.1 and claims that it will be alkaline when dissolved, while another participant suggests that the neutral alanine in solution is zwitterionic.
- Participants discuss the relationship between the pI of alanine and its neutral form, with some confusion about the basicity of the alaninate salt and the role of the carboxyl group in accepting protons.
- There is speculation about the polymerization of alanine in water, with some participants suggesting that the carboxyl group acts as a weak acid, while others clarify that polymerization is unlikely to occur under normal conditions.
- One participant raises the question of whether zwitterionic amino acids have the NH2 group acting as a weak base and the COOH group as a weak acid, leading to a discussion about the relative strengths of these groups affecting the overall pH.
Areas of Agreement / Disagreement
Participants express differing views on the behavior of alanine salt in solution, particularly regarding its pH and the roles of its functional groups. There is no consensus on whether the salt is acidic or basic, and the discussion remains unresolved on several technical points.
Contextual Notes
Some participants indicate a need for a better understanding of the ionization of amines and carboxylic acids, suggesting that assumptions about the behavior of alanine and its salt may depend on specific definitions and conditions not fully explored in the discussion.
 .
.