Unart
- 25
- 0
- Homework Statement
- 79. Chlorine gas reacts with fluorine gas to form chlorine trifluoride.
Cl2( g) + 3 F2( g) = 2 ClF3( g)
A 2.00-L reaction vessel, initially at 298 K, contains chlorine gas at a partial pressure of 337 mmHg and fluorine gas at a partial pressure of 729 mmHg. Identify the limiting reactant and determine the theoretical yield of ClF3 in grams.
- Relevant Equations
- PV=nTR
where
P=pressure
V=Volume
R= gas constant
n= moles
T = temperature in kelvins
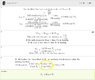
So essentially I followed pretty much what is in the picture
here in this solution.
https://www.slader.com/textbook/9780134162430-chemistry-a-molecular-approach-4th-edition/242/exercises/79/
My question is essentially why does he do the 0.07XXX mol f2 * 2mol clf3/3mol f2 * 92xx g/2mol f2 = answerfor the weight. I always thought as the mole to grams as a 1 for 1 sort of deal. That is if something has a mass of 95.45 amus that means that 1 mole weighs ~ 95.45 grams. However, he divided by 2 again where one would normally not because you are essentially dividing mass per mol by 2, making the molecule lighter than it really is.
here in this solution.
https://www.slader.com/textbook/9780134162430-chemistry-a-molecular-approach-4th-edition/242/exercises/79/
My question is essentially why does he do the 0.07XXX mol f2 * 2mol clf3/3mol f2 * 92xx g/2mol f2 = answerfor the weight. I always thought as the mole to grams as a 1 for 1 sort of deal. That is if something has a mass of 95.45 amus that means that 1 mole weighs ~ 95.45 grams. However, he divided by 2 again where one would normally not because you are essentially dividing mass per mol by 2, making the molecule lighter than it really is.