- #1
duchuy
- 79
- 3
- Homework Statement
- x
- Relevant Equations
- 2 mechanisms proposed below
Hi,
I have a question regarding these 2 possibilities: Why does the addition of RMgX on an ester always form a ketone? We could very well have that tetrahedral intermediate without eliminating the alcoolate group no? Since your reaction pretty much always tend towards the formation of a weaker base, the second reaction goes from carbanion like molecule to a neutral molecule, whilst the first textbook way would form an alcoolate?
Please explain to me what is the motor for the 1st reaction so that the reaction would preferably go that way instead of the second one.
Thank you so much for your help.
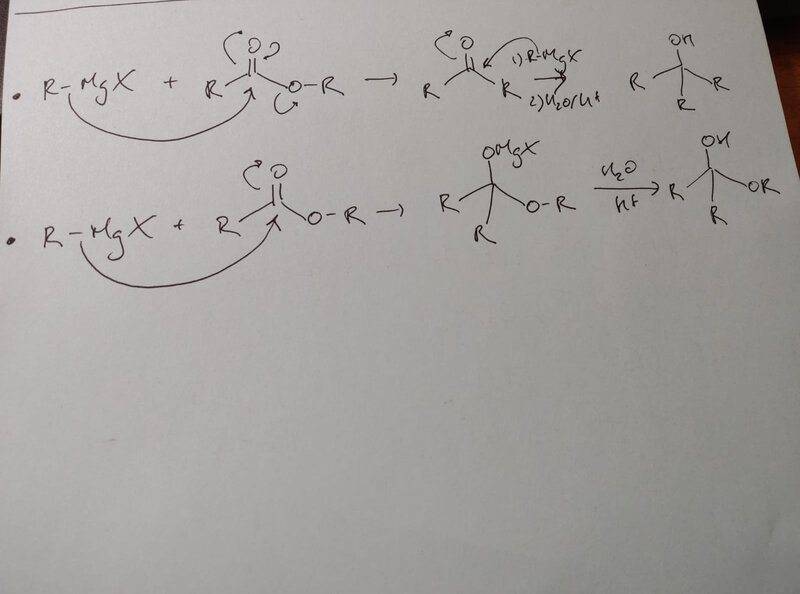
I have a question regarding these 2 possibilities: Why does the addition of RMgX on an ester always form a ketone? We could very well have that tetrahedral intermediate without eliminating the alcoolate group no? Since your reaction pretty much always tend towards the formation of a weaker base, the second reaction goes from carbanion like molecule to a neutral molecule, whilst the first textbook way would form an alcoolate?
Please explain to me what is the motor for the 1st reaction so that the reaction would preferably go that way instead of the second one.
Thank you so much for your help.