- #1
dRic2
Gold Member
- 883
- 225
Hi, PF
I have to comment this graphic about a reaction with and without catalysis.
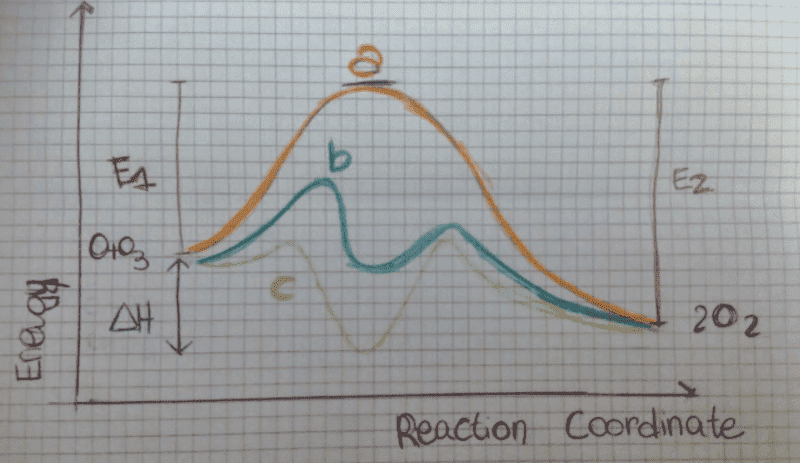
It's clear that the 'b' and 'c' line represent the catalysed processes, but I don't get one thing: my professor said that 'c' is a worse catalyst than 'b'. Why?
Thank you
I have to comment this graphic about a reaction with and without catalysis.
It's clear that the 'b' and 'c' line represent the catalysed processes, but I don't get one thing: my professor said that 'c' is a worse catalyst than 'b'. Why?
Thank you
