- #1
cuallito
- 95
- 1
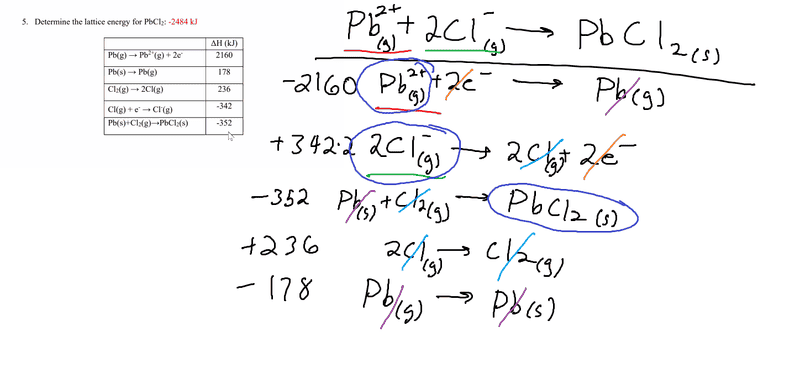
- Homework Statement
- Determine the lattice energy of PbCl2
- Relevant Equations
- Lattice energy= Heat of formation- Heat of atomization- Dissociation energy- (sum of Ionization energies)- (sum of Electron Affinities)
Here's my attempt at solving it using Hess's Law. I get -1770kJ when I add up all the numbers. The correct answer is supposed to be -2484 kJ.