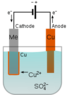- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Electrolysis of Water: How Does the Electric Field Affect Covalent Bonds?
- Thread starter erocored
- Start date
-
- Tags
- Electrolysis Work
In summary: Electrolysis is quite a complicated kinetic process. Maybe it would be better to first study and understand the simpler case with ideal polarised electrodes, where you have no charge transfer across the metal-solution interface and as such this interface can be treated as a capacitor.
Physics news on Phys.org
- #2
Frigus
- 337
- 160
I am not able to understand this statement.erocored said:What does break bounds in CuSO4?
I am interpreting this as "why does CuSO₄ hydrolyse?"
It is a salt and they dissociate in H₂O as they get more stable after dissociating.
Because initially atoms of right electrode have equal protons and electrons and when electrons leave the atom it became positively charged(atom).erocored said:And why does the right electrode getting a positive charge?
- #3
erocored
- 30
- 7
I meant why CuSO4 does split into Cu and SO4?Hemant said:I am not able to understand this statement.
- #4
Frigus
- 337
- 160
I have tried to answer this question in post #2.erocored said:I meant why CuSO4 does split into Cu and SO4?
- #5
Merlin3189
Homework Helper
Gold Member
- 1,730
- 818
erocored said:What does break bounds in CuSO4?
Yes. I was going just to say, "water"!Hemant said:It is a salt and they dissociate in H₂O as they get more stable after dissociating.
##Cu^{++} and \ SO_4^{--} ## obviously are attracted and need some energy to break them apart. But once they are surrounded by water they return more than that energy by binding to the water molecules. ##Cu^{++} ## attracts 6 water molecules. ##SO_4^{--} ## attacts up to 12 water molecules.
So your solution already contains the (hydrated) ##Cu^{++} and \ SO_4^{--} ## ions, free to move in solution.
The ##Cu^{++} ## ions are attracted to the -ve electrode, increasing their concentration there, and they are repelled from the +ve electrode, reducing the concentration there.
Simply because the battery is there.erocored said:And why does the right electrode getting a positive charge?
When ##Cu ## atoms dissolve to become ##Cu^{++} ## ions, it leaves behind electrons, which would actually cause the electrode to become negatively charged. But the battery removes them - or makes them move through the wires and go to the left electrode.
The negative charge on the left elctrode (caused by the battery) attracts the ##Cu^{++} ## ions and supplies the electrons needed to convert them to ##Cu ## atoms on its surface.
It doesn't ! CuSO4 is actually crystals of ##Cu^{++} \ ions, \ and \ SO_4^{--} \ ions \ ## which are electrostatically attracted together. The water enables the ions to separate, as explained at the start.erocored said:I meant why CuSO4 does split into Cu and SO4?
- #6
etotheipi
Hemant said:Because initially atoms of right electrode have equal protons and electrons and when electrons leave the atom it became positively charged(atom).
I don't think this is quite correct; the electrodes are metals whose electronic structure is like an 'electron sea', i.e. there is an overlap of the valence and conduction bands, and these bands are filled up to the Fermi level.
In vague terms, when you apply a voltage across an electrolytic cell, on one side of the cell the Fermi level of the electrode exceeds the energy of the lowest unoccupied molecular orbital (LUMO) of the species in the solution which means that the free energy can be reduced by electron transfer into the solution [and vice versa for the other electrode and the highest occupied molecular orbital (HOMO) of the species in the solution].
In reality, electrolysis is quite a complicated kinetic process. Maybe it would be better to first study and understand the simpler case with ideal polarised electrodes, where you have no charge transfer across the metal-solution interface and as such this interface can be treated as a capacitor.
- #7
DaveE
Science Advisor
Gold Member
- 3,710
- 3,319
As others have said, you don't have to break the bonds of this salt, the electric field is just moving the ions apart.
However, for other molecules, you can break bonds with a strong enough electric field (i.e. the voltage between the electrodes). For example, if you only put water in the beaker you can break the bond between the H2 and O. H2O is a polar molecule (meaning one side has more electric charge than the other). So, if the field is strong enough, it will try to pull the side with more positive charge towards the cathode and also pull the more negative side towards the anode. If the e-field is strong enough this force can overcome the force of the covalent bond and separate it: 2H2O → 2H2 + O2.
edit: This doesn't only apply to polarized molecules. With a strong enough field you can rip apart anything that isn't an elementary particle.
However, for other molecules, you can break bonds with a strong enough electric field (i.e. the voltage between the electrodes). For example, if you only put water in the beaker you can break the bond between the H2 and O. H2O is a polar molecule (meaning one side has more electric charge than the other). So, if the field is strong enough, it will try to pull the side with more positive charge towards the cathode and also pull the more negative side towards the anode. If the e-field is strong enough this force can overcome the force of the covalent bond and separate it: 2H2O → 2H2 + O2.
edit: This doesn't only apply to polarized molecules. With a strong enough field you can rip apart anything that isn't an elementary particle.
- #8
Frigus
- 337
- 160
Thanks for pointing this out.etotheipi said:I don't think this is quite correct; the electrodes are metals whose electronic structure is like an 'electron sea', i.e. there is an overlap of the valence and conduction bands, and these bands are filled up to the Fermi level.
In vague terms, when you apply a voltage across an electrolytic cell, on one side of the cell the Fermi level of the electrode exceeds the energy of the lowest unoccupied molecular orbital (LUMO) of the species in the solution which means that the free energy can be reduced by electron transfer into the solution [and vice versa for the other electrode and the highest occupied molecular orbital (HOMO) of the species in the solution].
In reality, electrolysis is quite a complicated kinetic process. Maybe it would be better to first study and understand the simpler case with ideal polarised electrodes, where you have no charge transfer across the metal-solution interface and as such this interface can be treated as a capacitor.
My teacher always says me to stop making my own theories but I always do it unknowingly
- #9
etotheipi
DaveE said:However, for other molecules, you can break bonds with a strong enough electric field (i.e. the voltage between the electrodes). For example, if you only put water in the beaker you can break the bond between the H2 and O. H2O is a polar molecule (meaning one side has more electric charge than the other). So, if the field is strong enough, it will try to pull the side with more positive charge towards the cathode and also pull the more negative side towards the anode. If the e-field is strong enough this force can overcome the force of the covalent bond and separate it: 2H2O → 2H2 + O2.
I don't believe this is really what happens, I think a few things are being mixed up here.
On the one hand you have self-ionisation of water, which is an equilibrium process ##\mathrm{{2H_2O}_{(l)} \rightleftharpoons {H_3O^+}_{(aq)} + {OH^-}_{(aq)}}## with ##\mathrm{K_w = [{H_3O^+}_{(aq)}][{OH^-}_{(aq)}] / (c^o)^2} \approx 10^{-14}## at R.T.P. But this is a very small effect, and these low concentrations are basically why you cannot electrolyse pure water easily [people have achieved electrolyte-free, pure water electrolysis using nanogap electrochemical cells - although that's quite different to what we're talking about here!]
You can use water containing an electrolyte to increase the efficiency of water-electrolysis. The half equations are sometimes quoted with ##\mathrm{H^+}##, but these are simplifications of the mechanism since isolated protons do not actually exist in solution - they are always hydronium ##\mathrm{H_3O^+}## ions. The proper half equations are,$$\mathrm{6H_2O_{(l)} \rightarrow {O_2}_{(g)} + {4H_3O^+}_{(aq)} + 4e^-}$$at the anode and$$\mathrm{{2H_2O}_{(l)} + 2e^- \rightarrow {H_2}_{(g)} + {2OH^-}_{(aq)}}$$at the cathode. At no point during this process does the electric field between the electrodes rip apart any covalent bonds. What happens really is that the hydronium ions migrate via the Grotthuss mechanism and one of the protons adsorbs to the cathode, is reduced to a ##\mathrm{H^{\bullet}}## radical and reacts with another ##\mathrm{H^{\bullet}}## radical to form ##\mathrm{H_2}##. The anode half equation is a bit more complicated, but that essentially involves another form of migration followed by the oxidation of ##\mathrm{OH^-}## to molecular oxygen.
Overall you do have a cell reaction ##\mathrm{{2H_2O}_{(l)} \rightarrow {O_2}_{(g)} + {2H_2}_{(g)}}##, but I don't think it's really got anything to do with the electric field splitting covalent bonds.
Last edited by a moderator:
1. What is electrolysis?
Electrolysis is a chemical process that involves using an electric current to break down a compound into its individual elements. It is commonly used to extract metals from their ores or to produce certain chemicals.
2. How does electrolysis work?
In electrolysis, an electric current is passed through an electrolyte solution, which contains ions. The ions are attracted to the electrodes, which are connected to a power source. At the negative electrode (cathode), positively charged ions gain electrons and are reduced, while at the positive electrode (anode), negatively charged ions lose electrons and are oxidized. This results in the separation of the elements present in the electrolyte.
3. What factors affect the efficiency of electrolysis?
The efficiency of electrolysis depends on several factors, including the concentration of the electrolyte solution, the strength of the electric current, the type of electrodes used, and the duration of the electrolysis process. Higher concentrations of electrolyte and stronger currents generally lead to more efficient electrolysis.
4. What are some practical applications of electrolysis?
Electrolysis has many practical applications, such as the production of metals like aluminum and copper, the production of chlorine gas for water treatment and the manufacture of plastics, and the extraction of hydrogen for use as a fuel source. It is also used in the production of batteries and in the purification of metals.
5. Are there any environmental concerns associated with electrolysis?
While electrolysis itself is a relatively clean process, the production of the electricity needed to power it can have environmental impacts. If the electricity is generated from fossil fuels, it can contribute to air pollution and greenhouse gas emissions. Additionally, some electrolytes used in the process can be harmful to the environment if not properly managed. However, there are efforts to use renewable energy sources and develop more environmentally friendly electrolytes for electrolysis processes.
Similar threads
-
Electromagnetism
- Replies
- 5
- Views
- 2K
-
Electromagnetism
- Replies
- 3
- Views
- 496
-
Electromagnetism
- Replies
- 1
- Views
- 642
-
Electromagnetism
- Replies
- 4
- Views
- 2K
-
Electromagnetism
- Replies
- 14
- Views
- 1K
-
Electromagnetism
- Replies
- 5
- Views
- 2K
-
Electromagnetism
- Replies
- 1
- Views
- 1K
-
Electromagnetism
- Replies
- 1
- Views
- 654
-
Biology and Chemistry Homework Help
- Replies
- 4
- Views
- 2K
-
Electromagnetism
- Replies
- 2
- Views
- 925
Share: