- #1
R Power
- 271
- 0
Hi friends
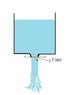
Consider a tank with a small nozzle at the bottom open to atmosphere. Water falls down through the nozzle as free jet as in figure attached . According to books pressure within this free jet at any point say point 1 will be equal to atmospheric pressure. This is because pressure at point 2 will be atmospheric and so at any cross sectional plane X-X pressure will be same, so at point 1 pressure will be atmospheric. Similarly, at any point within this free jet pressure will be atmospheric.
But if absolute pressure within free jet is atmospheric then the water should boil. Can u explain this.
Consider a tank with a small nozzle at the bottom open to atmosphere. Water falls down through the nozzle as free jet as in figure attached . According to books pressure within this free jet at any point say point 1 will be equal to atmospheric pressure. This is because pressure at point 2 will be atmospheric and so at any cross sectional plane X-X pressure will be same, so at point 1 pressure will be atmospheric. Similarly, at any point within this free jet pressure will be atmospheric.
But if absolute pressure within free jet is atmospheric then the water should boil. Can u explain this.