- #1
WWCY
- 479
- 12
Hi all, I have encountered the idea of a heat bath but am slightly perplexed as to what it is.
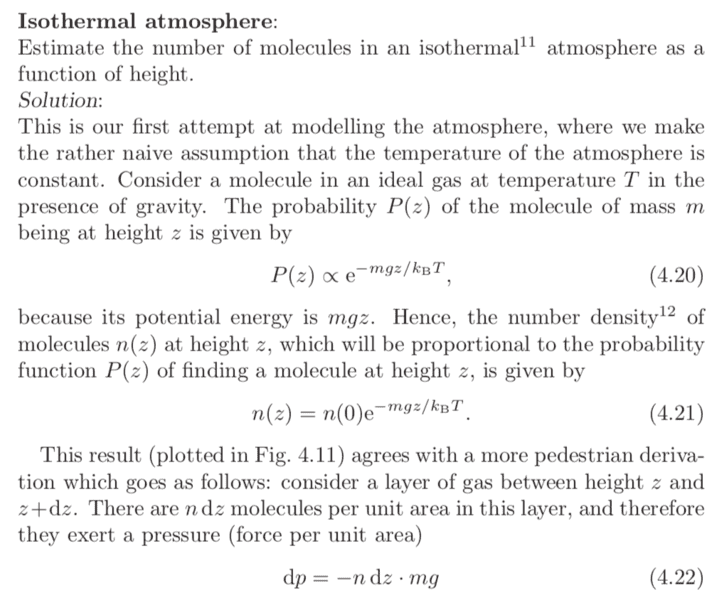
There was a textbook example that looked to find the number density expression for gas molecules as a function of position (image below). It then said that the probability ##P(z)## of finding the particle at height ##z## was given by
$$P(z) \propto e^{- mgz / k_B T}$$
a) Does this not mean that the particle is drawing gravitational potential energy from the heat bath? What sort of "object" would this heat bath be?
b) I might be being slightly pedantic here but isn't the probability of an event occurring at any point ##z## equal to ##0## for continuous distributions? If so, is the "proper" way of obtaining the expression to consider the probability in the interval ##[z, z+dz]##?
Many thanks in advance!
There was a textbook example that looked to find the number density expression for gas molecules as a function of position (image below). It then said that the probability ##P(z)## of finding the particle at height ##z## was given by
$$P(z) \propto e^{- mgz / k_B T}$$
a) Does this not mean that the particle is drawing gravitational potential energy from the heat bath? What sort of "object" would this heat bath be?
b) I might be being slightly pedantic here but isn't the probability of an event occurring at any point ##z## equal to ##0## for continuous distributions? If so, is the "proper" way of obtaining the expression to consider the probability in the interval ##[z, z+dz]##?
Many thanks in advance!
