- #1
Topher925
- 1,566
- 7
Very simple question but it has really been bothering me lately. Recently, a professor asked us this in class to which I said, "yes, of course it is" while everyone else said that it was not including the professor. He tried explaining it but I still don't understand.
We measure pressure by measuring a load created by a fluid, hence the units for pressure. However, for a pressure to exist a surface is not required. For example the pressure at the center of a balloon. Unless I'm mistaken, the pressure at the center of a balloon is the average force at which the gas particles interact with each other due to a change in their momentum from collisions. So practically the force would be better represented as the root mean square of all forces in all directions.
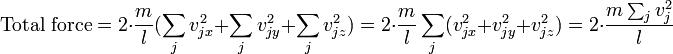
(from wikipedia)
Is this average force not equivalent to pressure, or do we have to define the molecular forces between molecules differently than pressure? In engineering we are taught to think of pressure as a load, but in chemistry we are taught to think of it as a state of matter. Which is it?
We measure pressure by measuring a load created by a fluid, hence the units for pressure. However, for a pressure to exist a surface is not required. For example the pressure at the center of a balloon. Unless I'm mistaken, the pressure at the center of a balloon is the average force at which the gas particles interact with each other due to a change in their momentum from collisions. So practically the force would be better represented as the root mean square of all forces in all directions.
(from wikipedia)
Is this average force not equivalent to pressure, or do we have to define the molecular forces between molecules differently than pressure? In engineering we are taught to think of pressure as a load, but in chemistry we are taught to think of it as a state of matter. Which is it?