- #1
Kaushik
- 282
- 17
Question 1
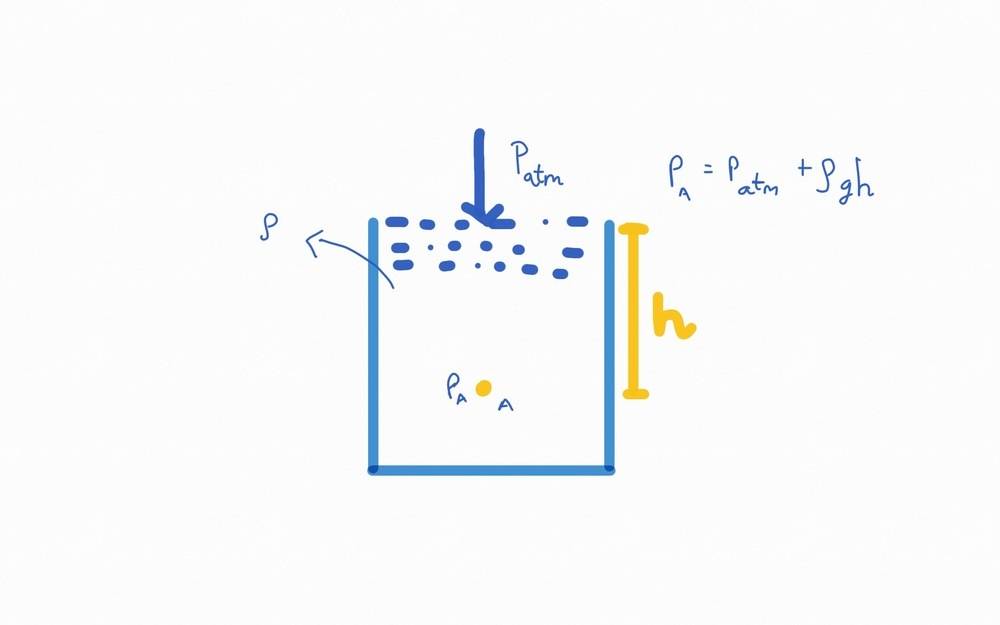
Consider a sample at a height ##ℎ## below the interface of air and the fluid. The pressure on the sample is given by ##𝑃_𝑎=𝑃_{𝑎𝑡𝑚}+𝜌𝑔ℎ##.
My question is does 𝑃𝑎 include the pressure due to the particles that are to the side of the sample?
Question 2
Now consider this setup.
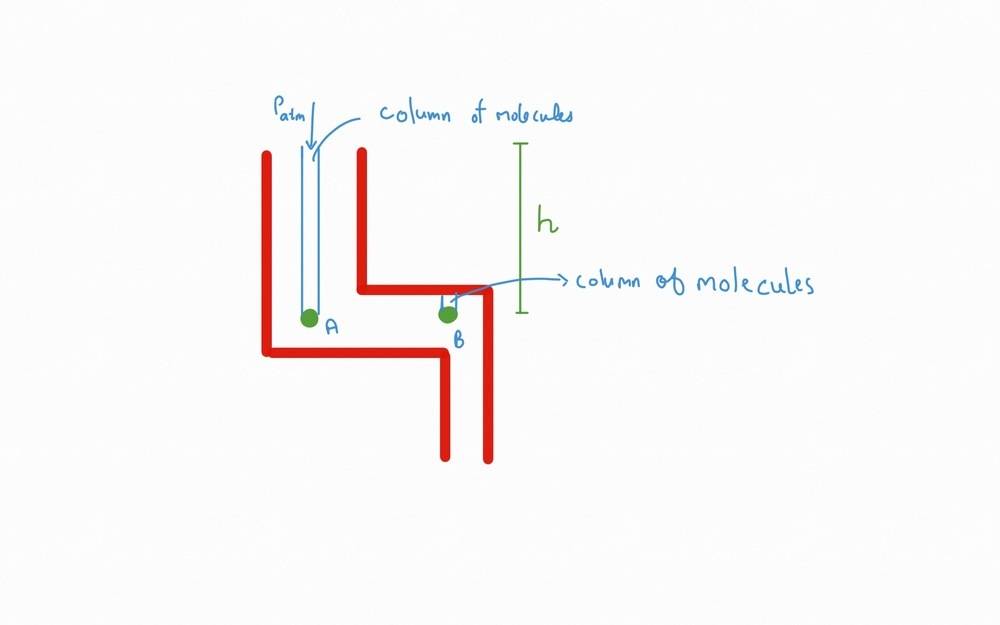
In this setup the two samples, say ##A## and ##B##, must experience same pressure i.e. ##P_a = P_b ## due to Pascals law. But the column of molecules above the sample is different. Isn't? Above B there are less number of molecules. On the other hand, above A there are more number of molecules and also ##P_{Atm}## adds to it. Due to this, the ##P_a > 𝑃_b##. But this isn't the case. When I asked this question to my teacher, he said that around the sample ##B## there are particles to the left that also exert pressure on it due to which ## P_a = P_b##
If this it true, then doesn't particles to the side of Sample ##A## also exert pressure on ##A##?
I asked the first question because of the second question.
Consider a sample at a height ##ℎ## below the interface of air and the fluid. The pressure on the sample is given by ##𝑃_𝑎=𝑃_{𝑎𝑡𝑚}+𝜌𝑔ℎ##.
My question is does 𝑃𝑎 include the pressure due to the particles that are to the side of the sample?
Question 2
Now consider this setup.
In this setup the two samples, say ##A## and ##B##, must experience same pressure i.e. ##P_a = P_b ## due to Pascals law. But the column of molecules above the sample is different. Isn't? Above B there are less number of molecules. On the other hand, above A there are more number of molecules and also ##P_{Atm}## adds to it. Due to this, the ##P_a > 𝑃_b##. But this isn't the case. When I asked this question to my teacher, he said that around the sample ##B## there are particles to the left that also exert pressure on it due to which ## P_a = P_b##
If this it true, then doesn't particles to the side of Sample ##A## also exert pressure on ##A##?
I asked the first question because of the second question.
