- #1
etotheipi
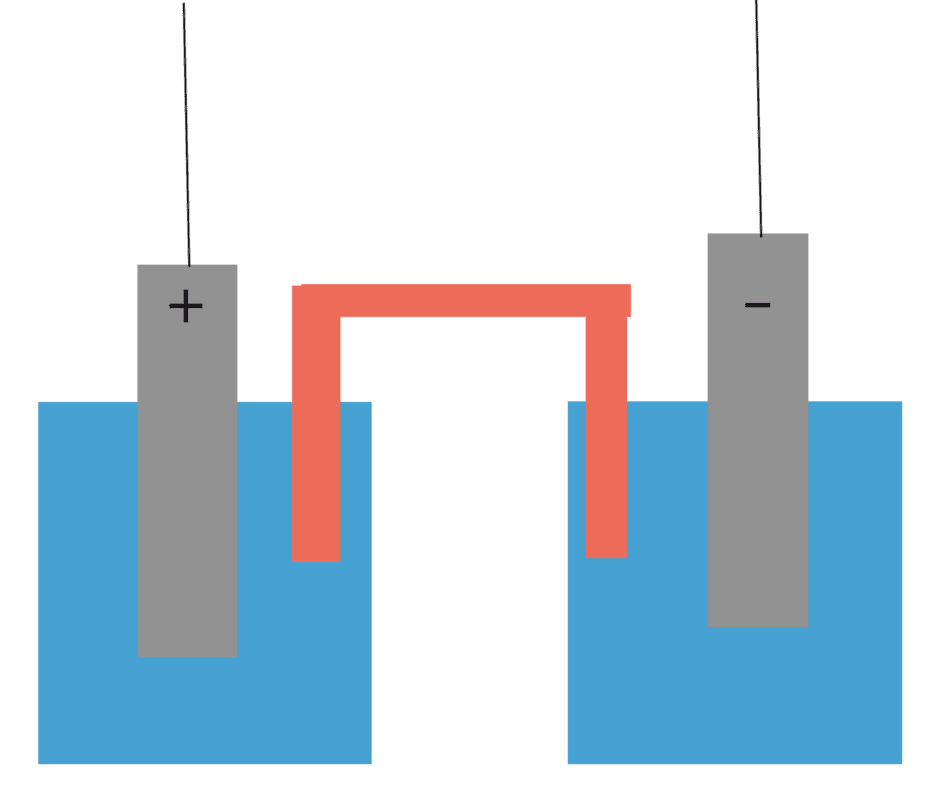
Suppose I have a galvanic cell, where I've arbitrarily set the (-) anode to have a potential of zero volts and the (+) cathode to ##\epsilon## V. The electrodes are connected via the load, but also via the solutions and salt bridge in the centre. Edit: The two trailing wires are connected to a load forming a complete circuit, though I haven't drawn them in.
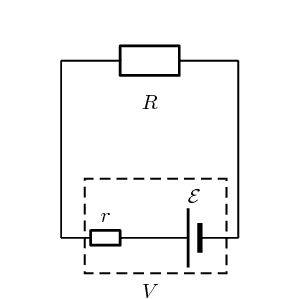
When idealised with a Thevenin equivalent in circuit theory, the internal resistance is put in series with the voltage source, and the current/voltage across the entirety of the practical cell can be modeled perfectly with this setup:
However, looking at the physical cell, the internal resistance appears as if it is directly between the two electrodes, resulting from the electrolyte and salt bridge. If the potential difference between the electrodes has magnitude ##\epsilon##, then it sort of seems like the current through the cell should be ##\frac{\epsilon}{r}## if we apply Ohms law between the electrodes within the cell. Evidently this cannot be a correct approach, since from the unambiguous theoretical setup we know that ##I = \frac{\epsilon}{R+r}##.
I was wondering where the mistake occurs when applying Ohms law between the electrodes. Is it perhaps that the internal resistance arises elsewhere? Thank you!
When idealised with a Thevenin equivalent in circuit theory, the internal resistance is put in series with the voltage source, and the current/voltage across the entirety of the practical cell can be modeled perfectly with this setup:
However, looking at the physical cell, the internal resistance appears as if it is directly between the two electrodes, resulting from the electrolyte and salt bridge. If the potential difference between the electrodes has magnitude ##\epsilon##, then it sort of seems like the current through the cell should be ##\frac{\epsilon}{r}## if we apply Ohms law between the electrodes within the cell. Evidently this cannot be a correct approach, since from the unambiguous theoretical setup we know that ##I = \frac{\epsilon}{R+r}##.
I was wondering where the mistake occurs when applying Ohms law between the electrodes. Is it perhaps that the internal resistance arises elsewhere? Thank you!
Last edited by a moderator: