- #1
Karagoz
Hi.
In physics articles, they say that sun light is white, and contain all colors.
But they show say that the Sun's atmosphere absorb light at certain wavelengths, causing the intensity of the light at this wavelength to drop and appear dark.
And the absorption spectrum looks like this:
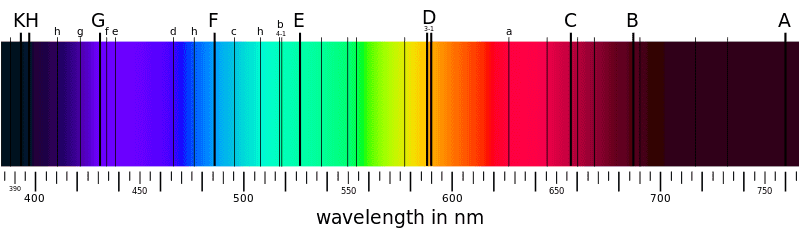
Does those black lines on the sun light spectrum show that the sunlight that reach the Earth doesn't contain all colors?
In physics articles, they say that sun light is white, and contain all colors.
But they show say that the Sun's atmosphere absorb light at certain wavelengths, causing the intensity of the light at this wavelength to drop and appear dark.
And the absorption spectrum looks like this:
Does those black lines on the sun light spectrum show that the sunlight that reach the Earth doesn't contain all colors?