- #1
K41
- 94
- 1
I don't understand, when calculating the force to change the surface area of the film, how the length obtained in the diagram below is "l" (lowercase L).
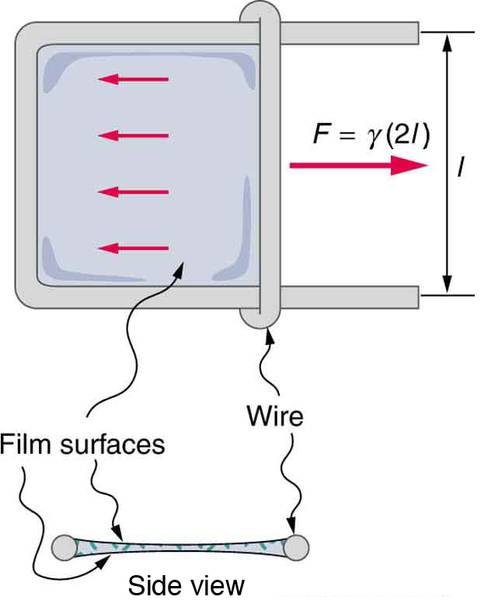 I understand that there are two surfaces to overcome, hence the factor of 2, but why is the length perpedicular to the force used and not the length parallel to the force, since surface tension is defined as the force parallel to a surface per unit length of that surface??
I understand that there are two surfaces to overcome, hence the factor of 2, but why is the length perpedicular to the force used and not the length parallel to the force, since surface tension is defined as the force parallel to a surface per unit length of that surface??
Last edited by a moderator: