cianfa72
- 2,808
- 298
Hi,
a basic doubt about thermodynamic functions and state variables. Take for instance transformations I and II in the following ##(p,V)## plane.
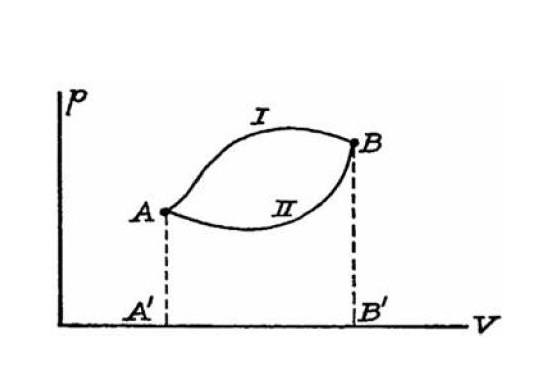
As far as I can tell, just because the transformations are drawn as continuous lines they are reversible by definition. Namely we can transform the system back in the other way and from a mathematical point of view it really makes sense (e.g. work and heat exchanged with the system's surrounding are the same as in the 'direct' transformation just with sign flipped).
By the way, given a system (e.g. a gas in a cylinder), there exist a pair of state variables for each way the system can exchange energy with the surrounding. In the above case the system can do work by means of gas thermodynamic pressure ##p## and exchange heat with the surrounding. Hence we have 2 pairs: ##(p,V)## and ##(t,S)## respectively.
Now my point is: how many are the independent state variables ?
The 'state equation' for the system involves the variables ##p,V,t## (take for instance the state equation of ideal gas) and I believe there exist another independent equation that involve the entropy ##S##. Thus in that case (system = gas in a cylinder) the independent state variables are just 2.
Does it makes sense ? Thank you.
Note: In the above I use lower case for intensive state variables and upper case the extensive ones.
a basic doubt about thermodynamic functions and state variables. Take for instance transformations I and II in the following ##(p,V)## plane.
As far as I can tell, just because the transformations are drawn as continuous lines they are reversible by definition. Namely we can transform the system back in the other way and from a mathematical point of view it really makes sense (e.g. work and heat exchanged with the system's surrounding are the same as in the 'direct' transformation just with sign flipped).
By the way, given a system (e.g. a gas in a cylinder), there exist a pair of state variables for each way the system can exchange energy with the surrounding. In the above case the system can do work by means of gas thermodynamic pressure ##p## and exchange heat with the surrounding. Hence we have 2 pairs: ##(p,V)## and ##(t,S)## respectively.
Now my point is: how many are the independent state variables ?
The 'state equation' for the system involves the variables ##p,V,t## (take for instance the state equation of ideal gas) and I believe there exist another independent equation that involve the entropy ##S##. Thus in that case (system = gas in a cylinder) the independent state variables are just 2.
Does it makes sense ? Thank you.
Note: In the above I use lower case for intensive state variables and upper case the extensive ones.
Last edited: