- #1
Afo
- 17
- 5
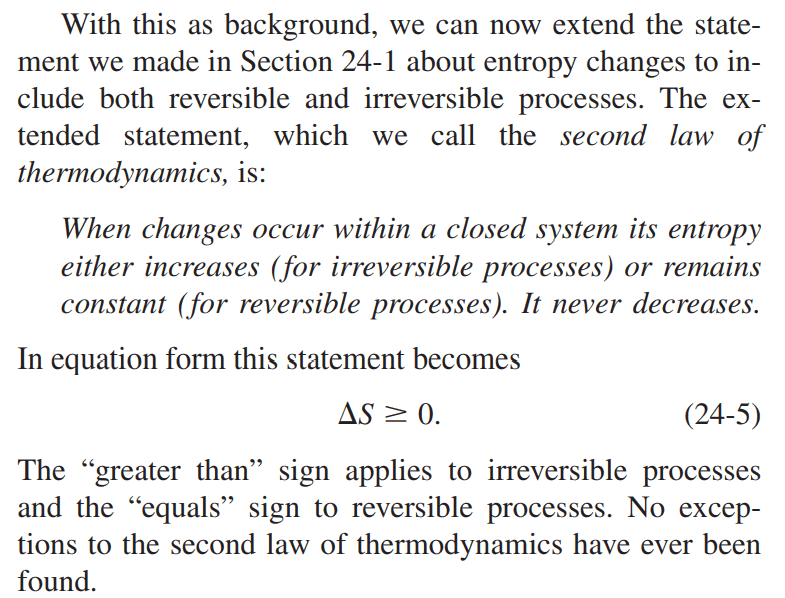
Homework Statement:: Why is the entropy of a closed system constant in a reversible process, and not related by ##\Delta S = \int_{i}^{f}\frac{dQ}{T}## (See below for the question in more details)
Relevant Equations:: ##\Delta S = \int_{i}^{f}\frac{dQ}{T}##
I am reading chapter 24 of Physics 1 by Halliday, Resnick, and Krane about the second law of thermodynamics and entropy.
Here's what I know:
1. A closed system is where there is no mass transfer between the environment, but there can be energy transfer as heat.
2. An isolated system is where there is no energy and mass transfer.
3. The entropy of a closed system increases in an irreversible process.

4. The change of entropy, ##\Delta S## for a reversible process in a closed system is defined as ##\Delta S = \int_{i}^{f}\frac{dQ}{T}##

The Question:
Relevant Equations:: ##\Delta S = \int_{i}^{f}\frac{dQ}{T}##
I am reading chapter 24 of Physics 1 by Halliday, Resnick, and Krane about the second law of thermodynamics and entropy.
Here's what I know:
1. A closed system is where there is no mass transfer between the environment, but there can be energy transfer as heat.
2. An isolated system is where there is no energy and mass transfer.
3. The entropy of a closed system increases in an irreversible process.
4. The change of entropy, ##\Delta S## for a reversible process in a closed system is defined as ##\Delta S = \int_{i}^{f}\frac{dQ}{T}##
The Question:
But then, the second law of thermodynamics states that the entropy remains constant for reversible processes. It's a contradiction since previously, the change in entropy was defined in the integral stated above.