- #1
another_dude
- 10
- 2
In school we are taught that sunlight contains all different frequencies of light. Also that each frequency has it's own unique wavelength and energy (per photon). So my question is that if there are infinitely many different wavelengths of light (much like infinitely many numbers in an interval) and each wavelength has it's own non-zero energy, how don't all these different and infinitely many energies add up to infinity?

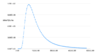
 , I decided to ignore it.
, I decided to ignore it.