Are Incorrect Bond Angles Common in Organic Chemistry?
- Thread starter alingy1
- Start date
-
- Tags
- Mistake
Click For Summary
Discussion Overview
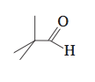
The discussion revolves around the accuracy of bond angles in organic chemistry representations, specifically in the context of the molecular structure of C5H10O. Participants explore whether it is common for bond angles to be misrepresented in organic chemistry drawings, particularly in projection formats.
Discussion Character
- Debate/contested
- Conceptual clarification
- Technical explanation
Main Points Raised
- Some participants question the accuracy of bond angles in the provided molecular representation of C5H10O, suggesting that it may not respect typical bond angles.
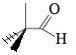
- One participant notes that the two methyl groups should be represented with a dash and a wedge to indicate a bond angle of 109.5 degrees, implying that the representation may be misleading.
- Another participant emphasizes that organic chemistry often uses projections that do not perfectly reflect three-dimensional structures, and that dashes and wedges are used to clarify spatial arrangements when necessary.
- There is a suggestion that the ambiguity in the representation may stem from the limitations of 2D drawings, which can lead to misinterpretations of bond angles.
Areas of Agreement / Disagreement
Participants express differing views on the commonality of incorrect bond angles in organic chemistry representations. While some acknowledge that inaccuracies can occur, others argue that such representations are generally accepted as long as the intended spatial arrangement is clear. The discussion remains unresolved regarding the extent to which bond angles are commonly misrepresented.
Contextual Notes
The discussion highlights limitations in 2D molecular representations, including potential ambiguities and the acceptance of non-ideal angles in projections. The reliance on visual cues like dashes and wedges is noted as a method to convey three-dimensionality, but the effectiveness of these cues can vary.
Similar threads
- · Replies 5 ·
- · Replies 4 ·
- · Replies 4 ·
- · Replies 1 ·
- · Replies 1 ·
- · Replies 4 ·
- · Replies 3 ·
- · Replies 1 ·
- · Replies 6 ·