GravityX
- 19
- 1
- Homework Statement
- Show that for a fluid column the chemical potential as a function of height can be written as follows ## \mu_h=\mu_0+mgh##
- Relevant Equations
- none
Hi
Unfortunately, I can't get on with the following task.
The system looks like this
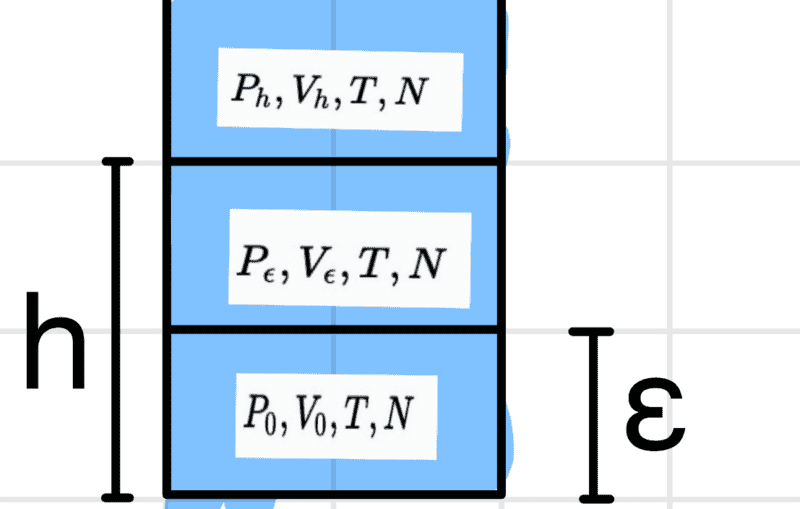
it is divided in such a way that the same number of particles is present in each ##\epsilon## section. I am now to determine the energy ##E(P_h,V_h,N)## at the height h using the energy ##h=0## i.e. ##E_0(P_h,V_h,N)## and with this I am then to derive the above equation for the chemical potential.
I would now have simply derived the energy using the hydrostatic pressure equation ##P=\rho gh+P_0## by simply multiplying the equation by the volume ##V_h##, i.e. ##PV_h=\rho ghV_h+P_0V_h=mgh+P_0V_h=U_h+U_0##.
Now I'm getting nowhere, unfortunately, because to get the chemical potential I would have to derive the energy according to N, so ##\mu=\frac{\partial U}{\partial N}## unfortunately there is no N in the above equation.
Unfortunately, I can't get on with the following task.
The system looks like this
it is divided in such a way that the same number of particles is present in each ##\epsilon## section. I am now to determine the energy ##E(P_h,V_h,N)## at the height h using the energy ##h=0## i.e. ##E_0(P_h,V_h,N)## and with this I am then to derive the above equation for the chemical potential.
I would now have simply derived the energy using the hydrostatic pressure equation ##P=\rho gh+P_0## by simply multiplying the equation by the volume ##V_h##, i.e. ##PV_h=\rho ghV_h+P_0V_h=mgh+P_0V_h=U_h+U_0##.
Now I'm getting nowhere, unfortunately, because to get the chemical potential I would have to derive the energy according to N, so ##\mu=\frac{\partial U}{\partial N}## unfortunately there is no N in the above equation.