salman213
- 301
- 1
Hi I was wondering if anyone can tell me how to find the bond order from a molecular orbital diagram.
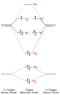
For example i found one on this website
attached...
"Comparison of the above energy level diagram wit hthat for nitrogen - you can see that the 2sg level lies lower than pu. Here, we are starting to fill the anti-bonding orbitals originating from the p orbital interactions and so the bond order decreases from three to two."
CAN someone give a quick explanantion.. thanks!
For example i found one on this website
attached...
"Comparison of the above energy level diagram wit hthat for nitrogen - you can see that the 2sg level lies lower than pu. Here, we are starting to fill the anti-bonding orbitals originating from the p orbital interactions and so the bond order decreases from three to two."
CAN someone give a quick explanantion.. thanks!