LT Judd
- 25
- 8
- TL;DR
- I am having trouble calculating the recoverable energy from a gas engine exhaust gas stream . Jenbacher J620 3000kw engine.My figures don't match the makers claims.
Hello,
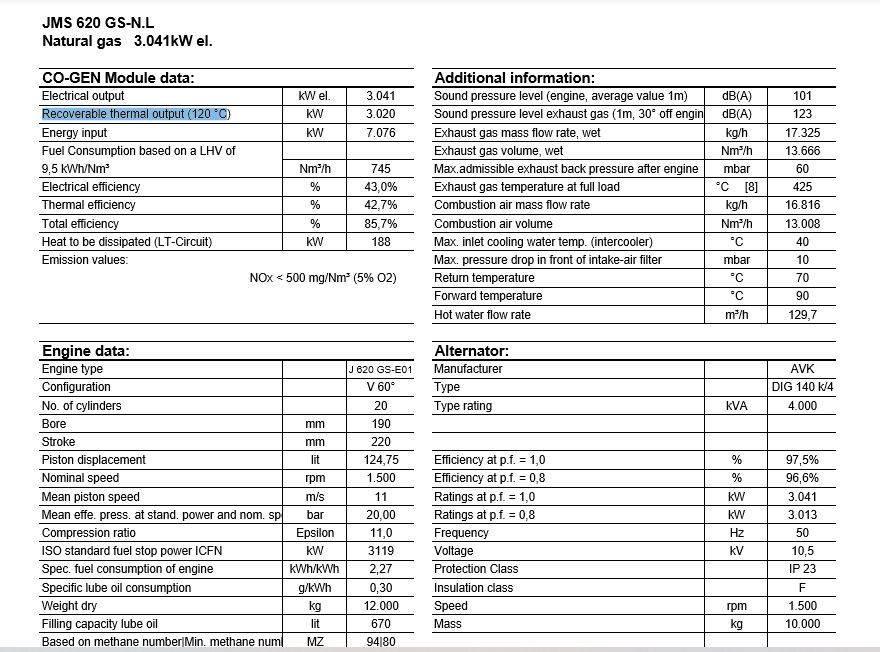
I am doing a study for my final years Uni project, which includes waste heat recovery from gas fuelled engine exhaust. I am using a data sheet for a Jenbacher J620 engine-.
In the data sheet it is stated that;
exhaust gas mass flow (wet) = 17325 kg hr ( not sure about the wet part?) this becomes 4.81 kg/s
exhaust gas temperature = 425 degC
recoverable thermal output (120 °C) = 3020 kw - I assume that means when exhaust gs is reduced to 120 deg C.
This gives an overall efficiency of 86% (thermal Plus electrical)
However when I apply the formula Qdot = mdot X CpX delta T
I get 4.81 X 1.15 X 305, which only comes to 1688 Kw , about half the claimed value !??
The claimed value doesn’t seem unreasonable because a review of the literature seems to show overall CHP efficiencies of 70-80% are the norm.
I did a mass weighting of different products of combustion of methane and 70% excess air to come up with that Cp,- a bit of a fudge, but its not that much difference to the Cp of straight air at 650K. The Cp would have to be about twice that to match the makers claimed figures for heat recovery.
I must be missing something- but what ??
 ??
??
I am doing a study for my final years Uni project, which includes waste heat recovery from gas fuelled engine exhaust. I am using a data sheet for a Jenbacher J620 engine-.
In the data sheet it is stated that;
exhaust gas mass flow (wet) = 17325 kg hr ( not sure about the wet part?) this becomes 4.81 kg/s
exhaust gas temperature = 425 degC
recoverable thermal output (120 °C) = 3020 kw - I assume that means when exhaust gs is reduced to 120 deg C.
This gives an overall efficiency of 86% (thermal Plus electrical)
However when I apply the formula Qdot = mdot X CpX delta T
I get 4.81 X 1.15 X 305, which only comes to 1688 Kw , about half the claimed value !??
The claimed value doesn’t seem unreasonable because a review of the literature seems to show overall CHP efficiencies of 70-80% are the norm.
I did a mass weighting of different products of combustion of methane and 70% excess air to come up with that Cp,- a bit of a fudge, but its not that much difference to the Cp of straight air at 650K. The Cp would have to be about twice that to match the makers claimed figures for heat recovery.
I must be missing something- but what ??