joaquinjbs
- 15
- 0
It's possible to calculate the latent heats of fusion and the specific heat of a solution of H2O and NaCl?
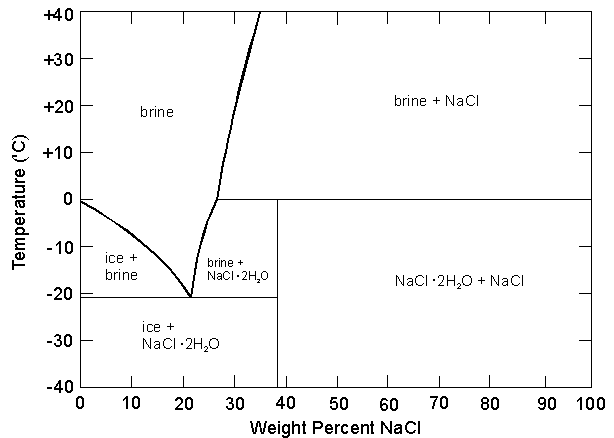
In some cases, it would have two latent heats of fusion? For example, at 10% NaCl, one at -21ºC and another at -5ºC?
Thanks!
In some cases, it would have two latent heats of fusion? For example, at 10% NaCl, one at -21ºC and another at -5ºC?
Thanks!