roam
- 1,265
- 12
I am trying to plot the absorption curve of a given sample using some spectrophotometric data. The data that I've collected are transmittance ##T## and reflectance ##R## – the ratios of transmitted and reflected light power to incident light power. However, using two different methods I got two very different answers:
(1) When radiation impinges on a material, we know that:
$$R+A+T=1 \tag{1}$$
Therefore, if we have obtained ##R## and ##T## using a spectrophotometer, then we can simply solve for ##A## and plot the results.
(2) Most textbooks define absorbance (sometimes called optical density, OD) as:
$$A=\log_{10}\left(\frac{1}{T}\right) \tag{2}$$
If we know ##T## using a spectrophotometer then we can plot absorption using Eq. 2.
Here are my results from both methods. The top curve is calculated using Eq. 1 (dashed/dotted lines are ##T## and ##R##). The bottom curve was calculated using Eq. 2 (but the answer had to be multiplied by 100 so that it appears as a percentage).
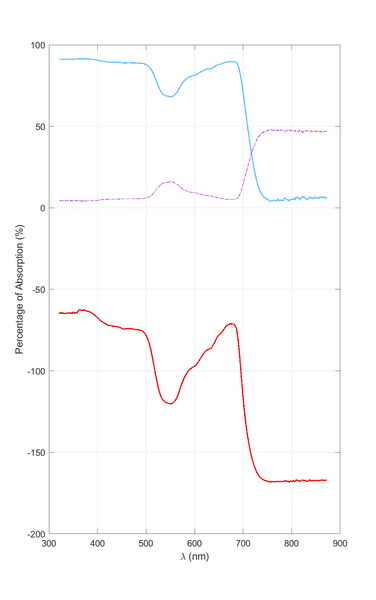
So, which is the correct method and why?
Also, using the second approach we get negative values for absorption (on the ordinate of the graph). How is this usually solved in the post-processing?
Any suggestions would be greatly appreciated.
(1) When radiation impinges on a material, we know that:
$$R+A+T=1 \tag{1}$$
Therefore, if we have obtained ##R## and ##T## using a spectrophotometer, then we can simply solve for ##A## and plot the results.
(2) Most textbooks define absorbance (sometimes called optical density, OD) as:
$$A=\log_{10}\left(\frac{1}{T}\right) \tag{2}$$
If we know ##T## using a spectrophotometer then we can plot absorption using Eq. 2.
Here are my results from both methods. The top curve is calculated using Eq. 1 (dashed/dotted lines are ##T## and ##R##). The bottom curve was calculated using Eq. 2 (but the answer had to be multiplied by 100 so that it appears as a percentage).
So, which is the correct method and why?
Also, using the second approach we get negative values for absorption (on the ordinate of the graph). How is this usually solved in the post-processing?
Any suggestions would be greatly appreciated.
