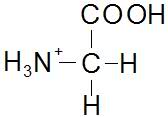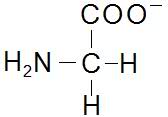SUMMARY
The discussion confirms the structures of Glycine at pH levels of 1, 6, and 13. At pH 6.1, Glycine exists as a Zwitterion, exhibiting both positive and negative charges that result in a net charge of zero. At low pH (1), Glycine is cationic, while at high pH (13), it becomes anionic. This information is crucial for understanding the behavior of Glycine in various pH environments.
PREREQUISITES
- Understanding of amino acid structures
- Knowledge of pH and its effects on molecular charge
- Familiarity with the concept of Zwitterions
- Basic principles of molecular biology
NEXT STEPS
- Research the isoelectric points of other amino acids
- Learn about the effects of pH on protein structure and function
- Explore the role of Zwitterions in biochemical reactions
- Study the implications of ionic forms of amino acids in drug design
USEFUL FOR
Molecular biologists, biochemists, and students studying protein chemistry will benefit from this discussion, particularly those interested in the behavior of amino acids under varying pH conditions.