SUMMARY
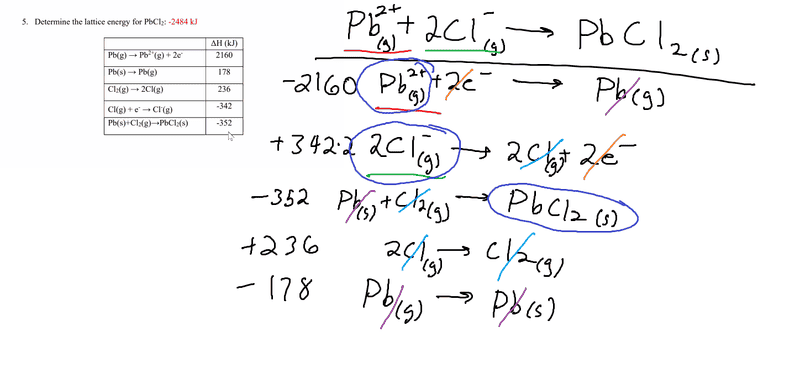
The discussion centers on the discrepancy in lattice energy calculations for lead(II) chloride (PbCl2) using Hess's Law. The user initially calculated a value of -1770 kJ but noted the correct value should be -2484 kJ. The error identified was the failure to flip the sign for the reaction 2Cl(g) → Cl2(g). Despite this correction, the user still could not reconcile their result with the book's value, suggesting potential inaccuracies in the textbook.
PREREQUISITES
- Understanding of Hess's Law
- Familiarity with lattice energy concepts
- Knowledge of thermodynamic calculations
- Basic chemistry principles related to ionic compounds
NEXT STEPS
- Review Hess's Law applications in thermodynamics
- Study lattice energy calculations for various ionic compounds
- Examine common errors in thermodynamic calculations
- Investigate discrepancies between textbook values and calculated results
USEFUL FOR
Chemistry students, educators, and professionals involved in thermodynamics and ionic compound analysis.