falcon999
- 1
- 0
- TL;DR
- I wish to understand if bubble formation in milk while being sloshed around, or the formation of separation layers will affect both the pressure and volume of the air head space above the milk
Summary: I wish to understand if bubble formation in milk while being sloshed around, or the formation of separation layers will affect both the pressure and volume of the air head space above the milk
Hi all : ) I have a basic physics question and sorry if its a very silly question:
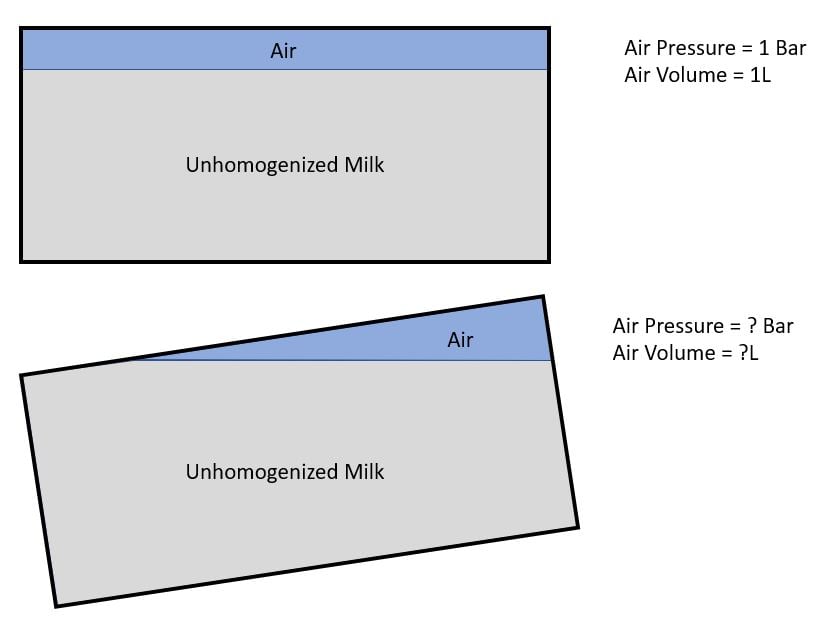
Let's say I have a sealed, air tight metal container shaped like a rectangular box, containing non-homogenized milk and a compressed air on top with a pressure of 1Bar and volume of 1L resting on a flat ground.
When the container is tilted at an angle (10 degrees), will there be any changes to the pressure and volume of the air on top?
My intuition told me that the air pressure and volume in an air tight container will remain constant regardless of the container's orientation. Is this a right assumption?
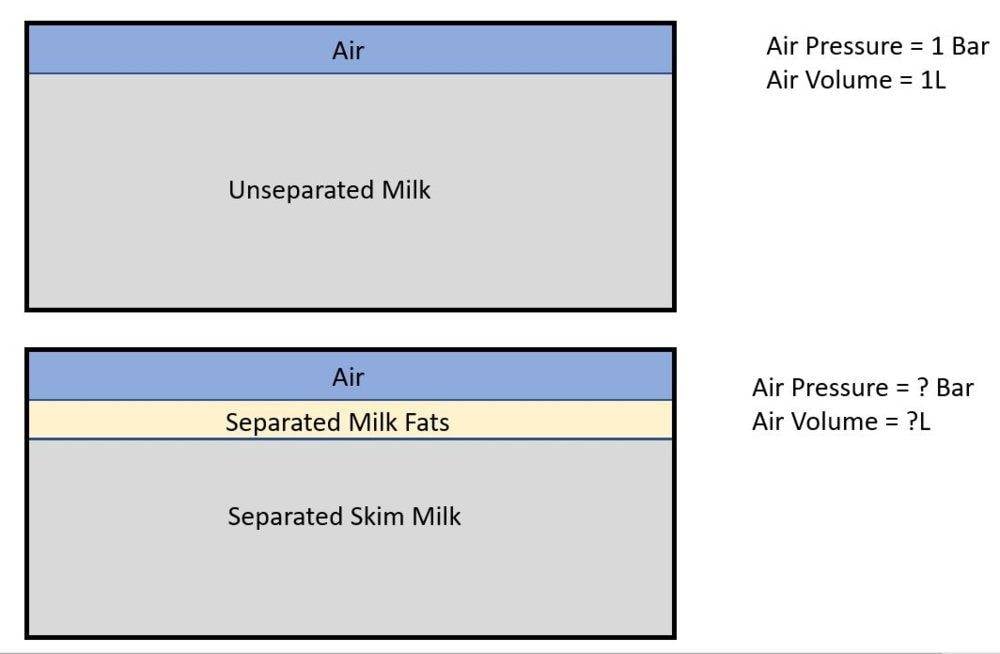
However, someone told me that when the containers are not stationary, the milk will produce 'bubbles" which will affect the air pressure and volume. Also, when the container is stationary, the milk fats will separate and form layers comprising of different ingredients and fat contents, which will also affect the pressure and volume, especially if the package is moved around AFTER such separation has taken place.
Can anyone able to provide me with some insights into whether this may be true?
If there are any effect on the air pressure and volume, if the container is huge with air volume of say 10000L, would it cause a significant changes that might arise safety concerns?
Thank you very much!
Wpu
Hi all : ) I have a basic physics question and sorry if its a very silly question:
Let's say I have a sealed, air tight metal container shaped like a rectangular box, containing non-homogenized milk and a compressed air on top with a pressure of 1Bar and volume of 1L resting on a flat ground.
When the container is tilted at an angle (10 degrees), will there be any changes to the pressure and volume of the air on top?
My intuition told me that the air pressure and volume in an air tight container will remain constant regardless of the container's orientation. Is this a right assumption?
However, someone told me that when the containers are not stationary, the milk will produce 'bubbles" which will affect the air pressure and volume. Also, when the container is stationary, the milk fats will separate and form layers comprising of different ingredients and fat contents, which will also affect the pressure and volume, especially if the package is moved around AFTER such separation has taken place.
Can anyone able to provide me with some insights into whether this may be true?
If there are any effect on the air pressure and volume, if the container is huge with air volume of say 10000L, would it cause a significant changes that might arise safety concerns?
Thank you very much!
Wpu