SUMMARY
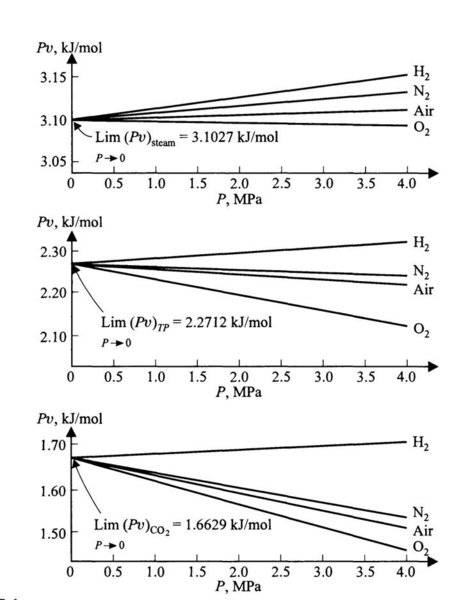
The discussion centers on the experimental virial equation for real gases, specifically the equation Pv = A(1 + BP) for small values of pressure (P). As pressure approaches zero, the product 'Pv' does not tend to zero due to the presence of the virial constants A and B, which introduce non-ideal behavior corrections. The conversation highlights that at low pressures, gases approximate ideal behavior, while deviations occur at higher pressures, necessitating the use of the virial expansion for accurate modeling. Reference is made to "Chemical Engineering Thermodynamics" by Smith & van Ness for further reading on this topic.
PREREQUISITES
- Understanding of the ideal gas law (PV = nRT)
- Familiarity with virial coefficients and their significance in gas behavior
- Basic knowledge of thermodynamics principles
- Experience with pressure-volume relationships in gases
NEXT STEPS
- Study the derivation and application of the virial equation in real gas scenarios
- Explore the concept of virial coefficients and their calculation methods
- Learn about deviations from ideal gas behavior at high pressures
- Read "Chemical Engineering Thermodynamics" by Smith & van Ness for in-depth understanding
USEFUL FOR
Students and professionals in chemical engineering, thermodynamics researchers, and anyone interested in the behavior of real gases under varying pressure conditions.