pranjal verma
- 13
- 1
Member advised to use the formatting template for all homework help requests
I am a high school student and currently studying Mechanical properties of fluid.
We are taught surface tension in a very introductory level and most of it is about liquid-gas surface tension.
We are taught that liquid-vapour tension is the atrractive forces that water molecules experience at the interface of liquid and gas but we are taught nothing about solid-liquid and solid-air surface tension.
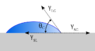
I the given diagram,I cannot understand why we are considering the surface tensions of solid - gas and solid - liquid interface.
- What will be the proper explanation of solid - liquid and solid - vapour surface tension?
- Why we are considering the surface tensions of solid- liquid interface and solid-gas interface when the system is water molecules?