firewatermax
- 1
- 0
Homework Statement
Could some one help me understand capillary Force?
Homework Equations
The Attempt at a Solution
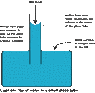
If we use a capillary tube sticking in a water cup as an example. The phenomenon will be water level is higher in the capillary tube than that in the water cup.
I understand that it is the surface tension force b/t tube and water which balances with the self-weight of the part of the water in the tube above the surrounding water level. But I just don't know why water would go up in the first place?
So just to make the question clear, I understand why water is able to stay in a higher level in the tube, but I don't know why it goes there.
Thanks for the help!
Attachments
Last edited: