CS Bence
- 12
- 0
Hey guys,
I'm trying to do some experiments at low temperatures in my garage. I have an air compressor that can push 6 cfm at 150 psi, and I want to expand this gas through an isentropic expander (NOT an isenthalpic expansion valve). Isentropic expansion extracts work from the fluid as it expands which cools the gas much much more than just expanding through a joule-thomson valve. I figure I can get 30 degC cooling in a work-producing expander, vs less than 2 degC in a joule-thomson valve.
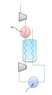
The attached image shows the process flow diagram for a simple reverse Brayton cycle. There is a counter current heat exchanger between the compressor and expander. The cold gas leaving the expander cools the gas that is entering the expander, and through this process we can get very cold temperatures at the turbine exit. (this is how some LNG processes work) I am in the process of designing the heat exchanger, which will likely be concentric tubes.
As you can imagine turbo-expanders do not exist commercially at low flowrates. Car/truck turbo-charger turbines require way too much flow for my application.
I want to modify an air compressor or a lawn mower internal combustion engine to become an expander.
Here are the problems I've identified so far...
Air compressor valves seem to all be passive, meaning one opens on the piston downstroke due to the pressure inside dropping, and the exhaust valve opens on the upstroke when the pressure inside rises. This is no good for trying to run this in reverse.
The internal combustion engine has valves connected to camshafts that are tied to the crankshaft, which is a good start. But the otto cycle has a compression stroke (upstroke with both inlet and exit valves closed), which is also no good.
I am assuming I need at least 3 piston/cylinders (120 degrees apart on the crankshaft) so that at all times one piston is receiving the force from the high pressure stream, keeping the crankshaft rotating.
So, I need to build/modify a piston/cylinder setup with camshafts/valves that simply opens one valve on a downstroke, and opens the other on the upstroke.
Any thoughts?
I'm trying to do some experiments at low temperatures in my garage. I have an air compressor that can push 6 cfm at 150 psi, and I want to expand this gas through an isentropic expander (NOT an isenthalpic expansion valve). Isentropic expansion extracts work from the fluid as it expands which cools the gas much much more than just expanding through a joule-thomson valve. I figure I can get 30 degC cooling in a work-producing expander, vs less than 2 degC in a joule-thomson valve.
The attached image shows the process flow diagram for a simple reverse Brayton cycle. There is a counter current heat exchanger between the compressor and expander. The cold gas leaving the expander cools the gas that is entering the expander, and through this process we can get very cold temperatures at the turbine exit. (this is how some LNG processes work) I am in the process of designing the heat exchanger, which will likely be concentric tubes.
As you can imagine turbo-expanders do not exist commercially at low flowrates. Car/truck turbo-charger turbines require way too much flow for my application.
I want to modify an air compressor or a lawn mower internal combustion engine to become an expander.
Here are the problems I've identified so far...
Air compressor valves seem to all be passive, meaning one opens on the piston downstroke due to the pressure inside dropping, and the exhaust valve opens on the upstroke when the pressure inside rises. This is no good for trying to run this in reverse.
The internal combustion engine has valves connected to camshafts that are tied to the crankshaft, which is a good start. But the otto cycle has a compression stroke (upstroke with both inlet and exit valves closed), which is also no good.
I am assuming I need at least 3 piston/cylinders (120 degrees apart on the crankshaft) so that at all times one piston is receiving the force from the high pressure stream, keeping the crankshaft rotating.
So, I need to build/modify a piston/cylinder setup with camshafts/valves that simply opens one valve on a downstroke, and opens the other on the upstroke.
Any thoughts?