- #1
Sorade
- 53
- 1
Hello all,
I am new to thermodynamics applied to turbines and compressors and I am trying to get my head around what is represented when calculating the work of a compression/expansion process using the polytropic exponent as oppose to the specific heat ratio of 1.4 (I'm working with air).
The attached image ( from Çengel, Y. a. (2004). Thermodynamics: An Engineering Approach. McGraw-Hill.)
Shows the equations for polytropic compression.
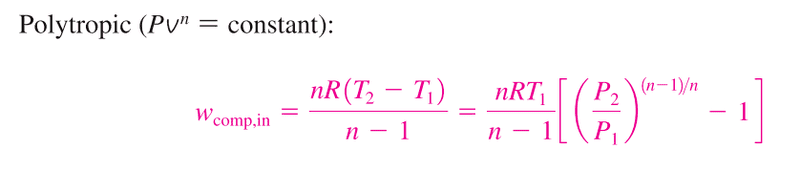
So I have two questions:
1) is the polytropic equation taking into account any heat losses compared to the following isentropic equation:
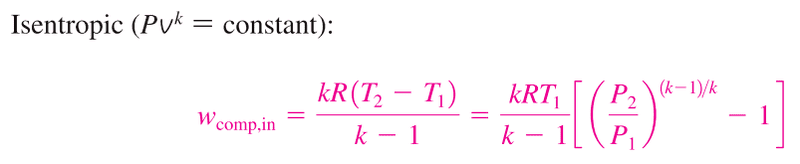
At the moment I am using the isentropic formulae and dividing it by the isentropic effciency which gives me a work value slightly higher accounting for irreversibilities.
2) What is the difference between using the polytropic exponent, n. Like in the first equation. And using the polytropic efficiency as below:
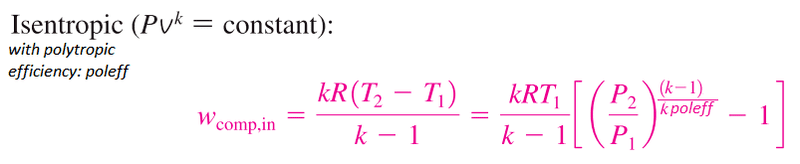
The reason I am asking is because using the polytropic exponent, n gives me greater efficiencies for my compression and expansion system than the ratio of specific heats, k. i.e lower compressor work and greater turbine work. Which I wouldn't expect if the polytopic exponent accounted for losses.
Thank you for your help.
I am new to thermodynamics applied to turbines and compressors and I am trying to get my head around what is represented when calculating the work of a compression/expansion process using the polytropic exponent as oppose to the specific heat ratio of 1.4 (I'm working with air).
The attached image ( from Çengel, Y. a. (2004). Thermodynamics: An Engineering Approach. McGraw-Hill.)
Shows the equations for polytropic compression.
So I have two questions:
1) is the polytropic equation taking into account any heat losses compared to the following isentropic equation:
At the moment I am using the isentropic formulae and dividing it by the isentropic effciency which gives me a work value slightly higher accounting for irreversibilities.
2) What is the difference between using the polytropic exponent, n. Like in the first equation. And using the polytropic efficiency as below:
The reason I am asking is because using the polytropic exponent, n gives me greater efficiencies for my compression and expansion system than the ratio of specific heats, k. i.e lower compressor work and greater turbine work. Which I wouldn't expect if the polytopic exponent accounted for losses.
Thank you for your help.