- #1
bakharuddin
- 5
- 2
I want to design a curding machine (Milk to Cheese) with an open system that operate at isobaric process (contant pressure),
P = 1 atm = 1,... bar
the range of temperature is = 25-75 0C
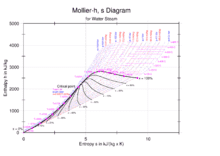
can i find an enthalpy of water from Mollier Diagram if my temp. is 25 to 75 degree celcius ? how ?
from my view, i must separates water properties from milk to make curds.
so i use mollier diagram but still confuse about it.
P = 1 atm = 1,... bar
the range of temperature is = 25-75 0C
can i find an enthalpy of water from Mollier Diagram if my temp. is 25 to 75 degree celcius ? how ?
from my view, i must separates water properties from milk to make curds.
so i use mollier diagram but still confuse about it.

