Tapias5000
- 46
- 10
Summary:: A nitric acid solution enters at a constant rate of 6 liters / minute into a large tank that originally contained 200 liters of a 0.5% nitric acid solution. The solution inside the tank is kept well stirred and leaves the tank at a rate of 8 liters / minute. If the solution entering the tank has 20% nitric acid, determine the volume of nitric acid in the tank after t minutes. When will the percentage of nitric acid in the tank reach 10%?
A nitric acid solution enters at a constant rate of 6 liters / minute into a large tank that originally contained 200 liters of a 0.5% nitric acid solution. The solution inside the tank is kept well stirred and leaves the tank at a rate of 8 liters / minute. If the solution entering the tank has 20% nitric acid, determine the volume of nitric acid in the tank after t minutes. When will the percentage of nitric acid in the tank reach 10%?
How do I solve the 10% question I got this far.
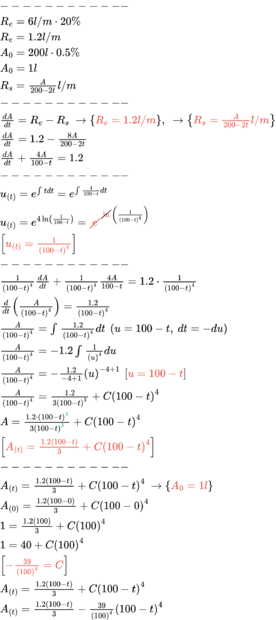
[Moderator's note: moved from a technical forum.]
A nitric acid solution enters at a constant rate of 6 liters / minute into a large tank that originally contained 200 liters of a 0.5% nitric acid solution. The solution inside the tank is kept well stirred and leaves the tank at a rate of 8 liters / minute. If the solution entering the tank has 20% nitric acid, determine the volume of nitric acid in the tank after t minutes. When will the percentage of nitric acid in the tank reach 10%?
How do I solve the 10% question I got this far.
[Moderator's note: moved from a technical forum.]
Last edited by a moderator: