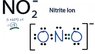
SUMMARY
The discussion focuses on calculating the formal charge in the Nitrite Ion (NO2-). Participants clarify the concept of valence electrons, stating that the total number of valence electrons for the Nitrite Ion is 18, derived from the contributions of nitrogen and oxygen atoms. The correct terminology is emphasized, correcting the initial misstatement of "violence electrons" to "valence electrons." Understanding formal charge calculation is essential for accurately representing molecular structures.
PREREQUISITES
- Understanding of formal charge calculation
- Knowledge of valence electrons in molecular structures
- Familiarity with the Nitrite Ion (NO2-) and its components
- Basic chemistry concepts related to ionic compounds
NEXT STEPS
- Research the formal charge calculation method for other polyatomic ions
- Study the Lewis structure representation of the Nitrite Ion
- Learn about resonance structures and their impact on formal charge
- Explore the role of formal charge in predicting molecular stability
USEFUL FOR
Chemistry students, educators, and anyone studying molecular structures and ionic compounds will benefit from this discussion.