- #1
JD_PM
- 1,131
- 158
I am studying how to determine the nuclear charge radius from direct measurement of the Coulomb energy differences of nuclei.
My book says that there is strong evidence which suggests that the nuclear force does not distinguish between protons and neutrons. Thus changing a proton into a neutron should not affect the nuclear energy of the system; only the Coulomb energy should change. Besides, it works out an easy example; transition from He to H; the energy difference between He and H is thus a measure of the Coulomb energy of the second proton, and the usual formula for the Coulomb repulsion energy can be used to calculate the distance between the protons and thus the size of the nucleus.
Then presents the difficulties of working with Uranium 238; 'in general it is not possible to calculate this effect to sufficient accuracy to be able to extract the Coulomb energy'. But then it starts focus on mirror nuclei pairs;
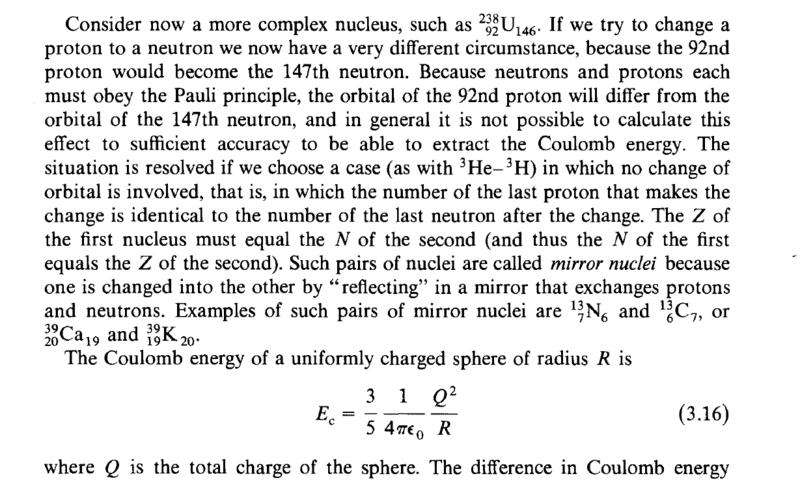
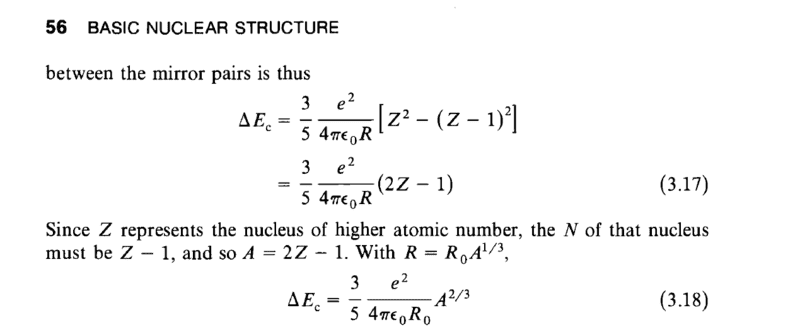
As you can see it derived a formula for energy differences on mirror nuclei but forgot about U; how could we explain the nature of the nuclear radius of Uranium 238 if we cannot do so by Coulomb's law?
I would like to discuss it using quantum mechanics.
My book says that there is strong evidence which suggests that the nuclear force does not distinguish between protons and neutrons. Thus changing a proton into a neutron should not affect the nuclear energy of the system; only the Coulomb energy should change. Besides, it works out an easy example; transition from He to H; the energy difference between He and H is thus a measure of the Coulomb energy of the second proton, and the usual formula for the Coulomb repulsion energy can be used to calculate the distance between the protons and thus the size of the nucleus.
Then presents the difficulties of working with Uranium 238; 'in general it is not possible to calculate this effect to sufficient accuracy to be able to extract the Coulomb energy'. But then it starts focus on mirror nuclei pairs;
As you can see it derived a formula for energy differences on mirror nuclei but forgot about U; how could we explain the nature of the nuclear radius of Uranium 238 if we cannot do so by Coulomb's law?
I would like to discuss it using quantum mechanics.
