- #1
b_andries
- 13
- 0
Hi,
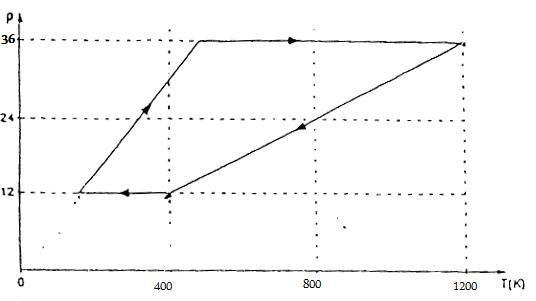
An amount of ideal gas undergoes the folowing cycle process in the following graph:
This process can be presented by the following PV-graph :
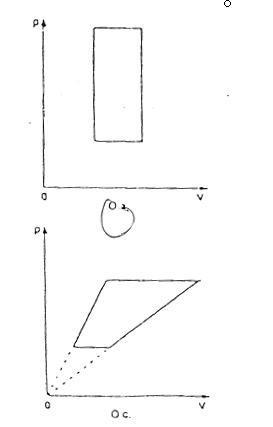
The answer has to be the upper graph but why can't it be the lower graph ?
Thank you!
An amount of ideal gas undergoes the folowing cycle process in the following graph:
This process can be presented by the following PV-graph :
The answer has to be the upper graph but why can't it be the lower graph ?
Thank you!

