tomtomtom1
- 160
- 8
- TL;DR
- Impact of Atmospheric Pressure on Water in a Tank and Pipe
Hello all
I was hoping someone could help with understanding how fluids level out under atmospheric pressure. For example:-
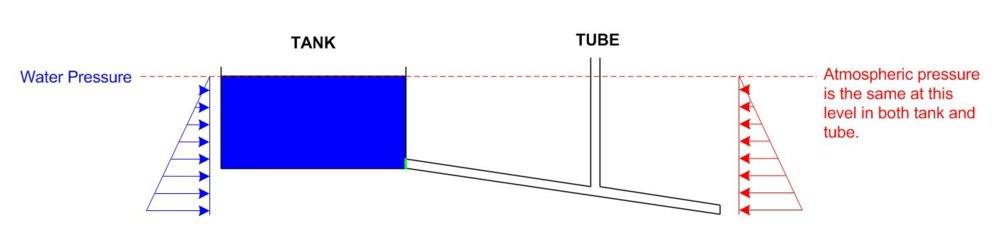
Below is a picture of a tank of water with a closed door at the bottom, the door leads to an inclined pipe that is closed off at the end there is another pipe connected vertically.
I have drawn arrows in red to represent the atmospheric pressure which gets greater due to depth and I have drawn arrows in blue to represent the water pressure which also gets greater due to depth.
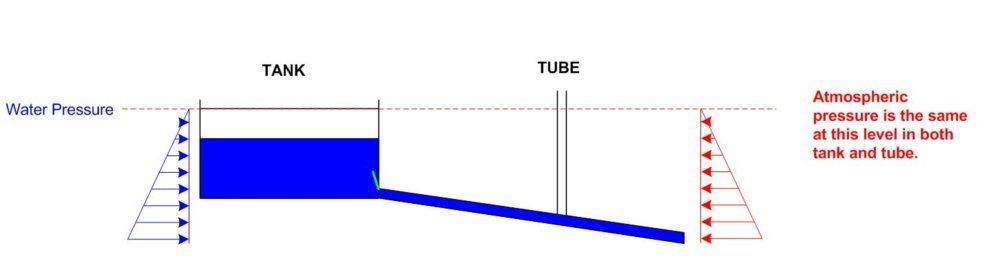
Now if i opened the green door and allowed the water to flow, but let's say for now the water did not rise up the tube then my diagram would look like:-
The question is why does the water rise up the tube and stop rising until the water in the tank and the water in the fluid are at the same level?
What i think is correct is that; as the water in the tank flows down into the pipe, the water level in the tank decreases which means that the force due to the pressure in the tank decreases but the atmosphere pressure in the tank increases.
But i am struggling to understand why the water would rise UP the tube when there is more atmospheric pressure pushing down?
Can anyone explain?
Thank you.
I was hoping someone could help with understanding how fluids level out under atmospheric pressure. For example:-
Below is a picture of a tank of water with a closed door at the bottom, the door leads to an inclined pipe that is closed off at the end there is another pipe connected vertically.
I have drawn arrows in red to represent the atmospheric pressure which gets greater due to depth and I have drawn arrows in blue to represent the water pressure which also gets greater due to depth.
Now if i opened the green door and allowed the water to flow, but let's say for now the water did not rise up the tube then my diagram would look like:-
The question is why does the water rise up the tube and stop rising until the water in the tank and the water in the fluid are at the same level?
What i think is correct is that; as the water in the tank flows down into the pipe, the water level in the tank decreases which means that the force due to the pressure in the tank decreases but the atmosphere pressure in the tank increases.
But i am struggling to understand why the water would rise UP the tube when there is more atmospheric pressure pushing down?
Can anyone explain?
Thank you.
Last edited: