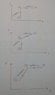RMalt
- 19
- 1
Homework Statement
[/B]
This is a very basic question. I have a cylinder filled with air having stops at a particular height .
First part of the process is isobaric as piston is free to move and P is constant.
When piston reaches stops this becomes isochoric process as volume is constant.
So far so good...
I need to sketch the T-V, P-V and P-T Diagrams on the same axis.
Homework Equations
Not sure of any equations that will help me in this, just knowledge of how Temperature, pressure and volume effect each other.
The Attempt at a Solution
I have drawn the P-v diagram as shown in the picture below. This is the easiest one to draw of all 3 as Pressure is constant in isobaric while volume is constant in isochoric
I know that in an isobaric process, T and V are directly proportional. I cannot find any information about T-V diagram from isochoric process and how these should be put together.
Also, I know that in an isochoric Process P and T are directly proportional. However I cannot understand how the P-T diagram for isobaric process will look like.
Unknown: T-V ---> isochoric and how this will look like when an isobaric process is added. (Check second picture for uploaded T-V diagram.
P-T ---> isobaric and how this will look when an isochoric process is added.
[/B]
Last edited: