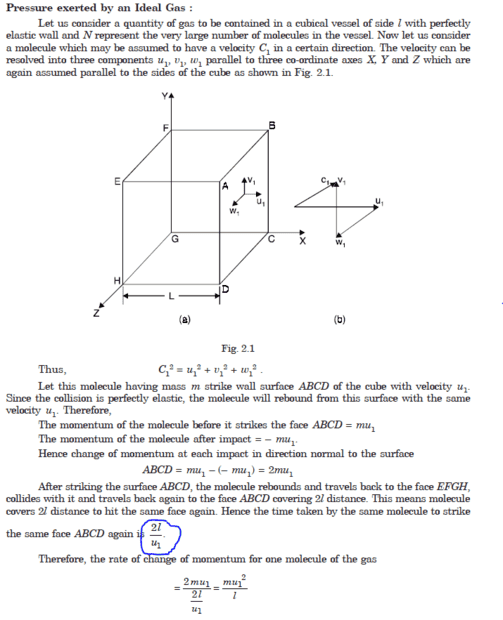
Vladimir_Kitanov
- 44
- 14
I understand everything except why do we use time between collisions instead of time of colission?
The discussion centers on the rationale for using the time between collisions rather than the time of collision in kinetic theory. The average rate of change of momentum for a molecule is determined by the momentum change during a collision divided by the time interval between collisions with a wall. This concept is analogous to calculating the average increase in a bank account based on the time between deposits. While introductory physics textbooks present this derivation, the erratic paths of gas molecules suggest the need for more sophisticated approaches found in statistical mechanics literature.
PREREQUISITESStudents of physics, researchers in thermodynamics, and anyone interested in the principles of kinetic theory and statistical mechanics.