Flucky
- 93
- 1
Edit: well this is frustrating, not sure why the itex things aren't working..
Edit2: I've attached a picture of the question at the bottom as well.
The cohesive energy of a solid noble gas may be written as
(See picture below)
Derive an expression for the equilibrium separation r0 of the atoms.
At Ecohesive, r=r0 and dE/dr0 = 0
So first I rearranged the main equation into something nicer to differentiate:
E = 6.065Br^{-12}_{0} - 7.225Ar^{-6}_{0}
Then differentiated:
dE/dr0 = -72.78\frac{B}{r^{13}_{0}} + 43.35\frac{A}{r^{7}_{0}} = 0
43.35\frac{A}{r^{7}_{0}} = 72.78\frac{B}{r^{13}_{0}}
r^{6}_{0} = \frac{72.78}{43.35}\frac{B}{A}
So r0 = (\frac{72.78}{43.35}\frac{B}{A})\frac{1}{6}
But this looks pretty horrible, is it any good?
Cheers.
-------------------------------------------------------------------------------------------
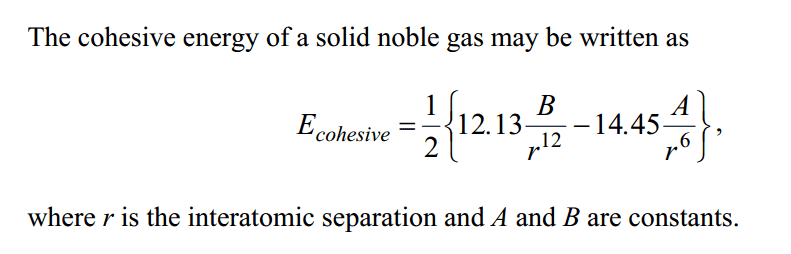
Edit2: I've attached a picture of the question at the bottom as well.
Homework Statement
The cohesive energy of a solid noble gas may be written as
(See picture below)
Derive an expression for the equilibrium separation r0 of the atoms.
The Attempt at a Solution
At Ecohesive, r=r0 and dE/dr0 = 0
So first I rearranged the main equation into something nicer to differentiate:
E = 6.065Br^{-12}_{0} - 7.225Ar^{-6}_{0}
Then differentiated:
dE/dr0 = -72.78\frac{B}{r^{13}_{0}} + 43.35\frac{A}{r^{7}_{0}} = 0
43.35\frac{A}{r^{7}_{0}} = 72.78\frac{B}{r^{13}_{0}}
r^{6}_{0} = \frac{72.78}{43.35}\frac{B}{A}
So r0 = (\frac{72.78}{43.35}\frac{B}{A})\frac{1}{6}
But this looks pretty horrible, is it any good?
Cheers.
-------------------------------------------------------------------------------------------
Last edited: